Tetrataenite Magnets
June 19, 2023
Iron is, by
mass, the
most common element on Earth, but iron
oxidizes (
rusts) rapidly, and
metallic iron is rarely found on
Earth's surface. Pure iron is a very
soft material with about the same
flexibility as
aluminum. However, addition of less than two percent
carbon produces
carbon steel, and some carbon steels are up to 1000 times
harder than pure iron. Another important
property of iron and some iron
alloys is their
ferromagnetism. Pure α-iron, the
room temperature body-centered cubic (BCC) crystal form of iron, is
magnetic below its
Curie temperature of 770
°C (1,420
°F; 1,040
K), and it can be made into a
permanent magnet by cooling through its Curie temperature in the presence of a
magnetic field.
Some iron alloys, in particular
Alnico 5, an alloy of iron (51 at-%), aluminum (8 at-%),
nickel (14 at-%),
cobalt (24 at-%), and
copper (3 at-%), were commonly used as permanent magnets in the
mid-20th century. One popular use was as
loudspeaker magnets. Such magnets were replaced by the superior
rare-earth magnets in the
1970s. An important
figure of merit of a magnet is its
maximum energy product, the maximal value of the
product of
magnetic induction (B) and
applied magnetic field (H) on its
hysteresis curve. The maximum energy product of Alnico 5 is 5.5 mega-
gauss-
oersted (MGOe, 43.8 k
J/
m3), while that of most
NdFeB rare earth magnets is greater than 60 MGOe (~500 kJ/m
3).
As I've written in several previous articles (including
Rare Earths from Coal Waste, April 18, 2022,
Rare Earth Metals from Fly Ash, July 7, 2016, and
Materials Supply Chain, July 14, 2014), most rare earth
mining and
refining is in
China. China has a near
monopoly on
global production, producing an estimated 60% of the world's rare earths in 2021, as compared with the 15% of the
United States.[1]
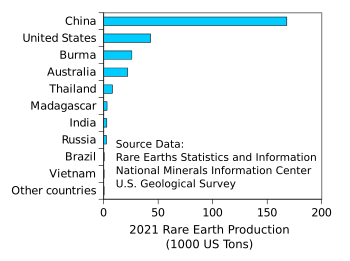
Estimated rare earth production by country in 2021.
China produced 168,000 US tons of rare earth oxide equivalent in 2021, up from 140,000 the previous year.
(Chart created using Gnumeric from data in ref. 2.[2] Click for larger image.)
A non-rare earth permanent magnet would be welcome, especially one made from
inexpensive elements such as iron.
Laboratory synthesis of an unique iron-nickel alloy with promising magnetic properties was announced at the end of 2022 in an
open access paper in
Advanced Science.[2-6] The alloy,
tetrataenite, is found in
meteorites, but rarely found in
nature. It was thought that it could be formed only by a very slow cooling process, estimated to be a few
degrees per million
years, that allowed
diffusion of
atoms into its
L10 layered crystal structure. Prior laboratory synthesis was achieved by
irradiation by
neutrons or
electrons that nudges its atoms into place.

A specimen of tetrataenite, recovered from Zacatecas, Mexico. This is a portion of an H5 chondrite meteor that fell on December 15, 1978. Its size is 2.7x2.0x2.0 centimeters. The tetrataenite crystals appear as silvery patches.
(Wikimedia Commons image by Robert M. Lavinsky.)
The 2022 study, by
materials scientists from the
Istituto Italiano di Tecnologia (Genova, Italy), the
Austrian Academy of Sciences (Leoben, Austria), the
Montanuniversität Leoben (Leoben, Austria), and the
University of Cambridge (Cambridge, UK) found that a small addition of
phosphorus allowed creation of tetrataenite by usual
smelting techniques.[2-6] Tetrataenite can be made permanently magnetic with a high
coercivity that's essential for maintaining a permanently magnetic state. It's
theoretical magnetic energy product is greater than 42 MGOe (335 kJ/m
3), nearly on par with rare earth magnets.[7] However, all theoretical predictions should be taken with
a grain of phosphorus until
experimentally proven.
The
research paper begins with a nice summary of the
prior art with information from a review by Wasilewski.[8] This is summarized in the following table.
Global demand for rare earth magnets is estimated to reach $37 billion by 2027.[6] Aside from China's putative control of the rare earth market, there are
environmental problems as well. Vast quantities of
ore need to be mined to extract small quantities of these elements.[4] Furthermore, the subsequent processing of the ore is environmentally
hazardous.[6] The tetrataenite research team was not intentionally on a quest to synthesize this material. They were doing research on the mechanical properties of glassy Fe-Ni-P-B alloys when they discovered dendrites in their specimens.[4-5] Careful measurements using
X-ray diffraction and
transmission electron microscopy revealed the L10 tetrataenite crystal phase.[4-5]
In their experiments, the alloying elements were
cast at 1123 K in copper
molds and then rapidly cooled at a rate of 10-10
4 K/sec.[2,5] Adjustment of the element
ratios showed that the rapid creation of the L10 phase was caused by the phosphorus.[5] Rods cast with a larger diameter, and therefore at a lower cooling rate, had a smaller
volume fraction of the dedritic tetrataenite.[2] The L10 phase had
lattice constants of a = 0.255±0.001 nm, and c = 0.363±0.001 nm.[2]

scanning electron micrograph of a lateral section of a 1 mm diameter rod of Fe50Ni30P13C7 showing tetrataenite dendrites in an alloy matrix.
(Fig. 1b of ref. 2, licensed under a Creative Commons License.)
Says study
author and
postdoctoral research associate at the University of Cambridge,
Yurii Ivanov, "The presence of phosphorus seems to be critical in permitting formation of tetrataenite without such treatments as neutron irradiation."[5] The role of phosphorous is to stabilize
vacancies in the
crystal lattice and accelerate atomic mobility.[2].
Calculations indicate that the atomic mobility at 573 K is accelerated by a factor of more than 10,000 by the addition of 1 at-% P.[2]
A
patent application has been filed, but the study authors did not attempt to create or characterize permanent magnets from their material.[2,4] Some experts see no prospect of these alloys replacing rare-earths in any permanent magnet application.[5] The Cambridge researchers agree that this alloy might not match high-performance neodymium-based magnets.[5] Says study author and
professor of
materials science at Cambridge,
Lindsay Greer,
"The analogy here would be that we have shown we can make a brick - a piece of tetrataenite - but not yet a house - a magnet."[5]
The research was
funded in part by the
European Research Council and the
Austrian Science Fund.[4]
References:
- Rare Earths Statistics and Information, National Minerals Information Center, U.S. Geological Survey, 2022.
- Yurii P. Ivanov, Baran Sarac, Sergey V. Ketov, Jürgen Eckert, and Lindsay A. Greer, "Direct Formation of Hard‐Magnetic Tetrataenite in Bulk Alloy Castings," Advanced Science, vol. 10, no. 1 (October 25, 2022), Article no. 2204315, doi:10.1002/advs.202204315. This is an open access article with a PDF file here.
- Laura H. Lewis, Ian J. Mcdonald, Sahar Keshavarz, and R. William McCallum, "Method of tetratenite production and system therefor," US Patent No. 11,462,358, October 4, 2022.
- New approach to 'cosmic magnet' manufacturing could reduce reliance on rare earths in low-carbon technologies, University of Cambridge Press Release, October 25, 2022.
- Sarah Wells, "Making Cosmic Magnets on Earth," Physics, vol. 15, no. 182 (November 23, 2022).
- Tanner Stening, "Accelerating the Production of Tetrataenite as Alternative to Rare-Earth Magnets," Northeastern University Press Release, October 7, 2022.
- E. Dos Santos, J. Gattacceca, P. Rochette, G. Fillion, and R.B. Scorzelli, "Kinetics of tetrataenite disordering," Journal of Magnetism and Magnetic Materials, vol. 375 (February 1, 2015), pp. 234-241, https://doi.org/10.1016/j.jmmm.2014.09.051.
- Peter Wasilewski, "Magnetic characterization of the new magnetic mineral tetrataenite and its contrast with isochemical taenite," Physics of the Earth and Planetary Interiors, vol. 52, no. 1-2 (October, 1988), pp. 150-158, https://doi.org/10.1016/0031-9201(88)90063-5.
Linked Keywords: Iron; mass; abundance of the chemical elements; most common element on Earth; oxide; oxidizes; rusts; metal; metallic; lithosphere; Earth's surface; ductility; soft; material; deflection (engineering); flexibility; aluminum; carbon; carbon steel; hardness; harder; physical property; alloy; ferromagnetism; room temperature (cientific use); body-centered cubic (BCC) crystal; magnetic; Curie temperature; Celsius; °C; Fahrenheit; °F; kelvin; permanent magnet; magnetic field; Alnico 5; nickel; cobalt; copper; mid-20th century; loudspeaker; rare-earth magnet; 1970s; figure of merit; maximum energy product; multiplication; product; magnetic induction (B); applied magnetic field (H); hysteresis curve; gauss (unit); oersted; joule; cubic meter; neodymium magnet; NdFeB; Rare Earths from Coal Waste; Rare Earth Metals from Fly Ash; Materials Supply Chain; mining; extractive metallurgy; refining; China; monopoly; world economy; global production; United States; 2021 World Rare Earth Production (estimated); rare earth element; mining; production; country; US tons; oxide; Gnumeric; cost; inexpensive; chemical element; laboratory; chemical synthesis; open-access journal; open access paper; Advanced Science; tetrataenite; meteorite; nature; Celsius; degrees; year; diffusion; atom; tetragonal crystal system; L10 layered crystal structure; irradiation; neutron; electron; Zacatecas, Mexico; H5 chondrite meteor; centimeter; crystal; silvery; Wikimedia Commons; Robert M. Lavinsky; materials science; materials scientist; Istituto Italiano di Tecnologia (Genova, Italy); Austrian Academy of Sciences (Leoben, Austria); Montanuniversität Leoben (Leoben, Austria); University of Cambridge (Cambridge, UK); phosphorus; smelting; coercivity; theory; theoretical; a grain of salt; experiment; experimentally; scientific journal; research paper; prior art; redox; oxidation and reduction; molecular beam epitaxy; diffusionless transformation; martensitic transformation; high pressure; torsion (mechanics); annealing (metallurgy); amorphous metal; glassy ribbon; rolling (metalworking); cold rolling; milling (grinding); melt spinning; melt-spun ribbon; nitriding; nanoparticle; nanopowder; environment (biophysical); environmental; ore; hazardous waste; X-ray crystallography; X-ray diffraction; transmission electron microscopy; casting; cast; mold; ratio; volume fraction; lattice constant; scanning electron microscope; scanning electron micrograph; diameter; cylinder (geometry); rod; dendrite (metal); alloy; composite material; matrix; Creative Commons License; author; postdoctoral research associate; Yurii Ivanov; vacancy defect; crystal structure; crystal lattice; calculation; patent application; professor; Lindsay Greer; analogy; brick; house; funding of science; funded; European Research Council; Austrian Science Fund.