Fondant Physics
August 7, 2023
There's a strong
connection between
cooking and
chemistry.
Chemists supposedly make good
cooks, since cooking, just like
chemical synthesis, involves selection of the correct
ingredients, careful
measurement of their quantities, selection of the right
utensils and
containment vessels, and processing
mixtures at the proper
temperature for the proper
time.
analytical chemists will carefully
sample the
product at stages and adjust things accordingly.
Cooking likewise entails some important
chemical reactions, such as the
Maillard reaction. This reaction between
amino acids imparts
flavor when certain
foods are
browned. This reaction flavors such foods as
breads,
biscuits and
French fries.
Pretzels are purposely coated with
lye to accelerate this reaction to give them a deep brown color.

"Do you want fries with that?"
(Photo by Evan-Amos, via Wikimedia Commons.)
Perhaps I'm
biased because of my profession, but I see much more
physics in a modern
household kitchen than chemistry. Going beyond the simple
mechanical devices, such as
can openers and
blenders, there are devices dependent on physical
principles. Every house has a
refrigerator that uses the
expansion of a
gas to cause
cooling by the well-known
thermodynamic process of
free expansion. Other cooling principles have been proposed to replace this current
technology. These include the
elastocaloric effect and the
magnetocaloric effect, as I've described in some previous articles (
Elastocaloric Effect, January 6, 2020,
Giant Magnetocaloric Effect, September 10, 2018, and
Magnetic Refrigeration, September 3, 2014).
The
Joule heating of the
resistance elements of
conventional ovens is a simple process that's been known since 1840, and the
transfer of heat from the resistance elements involves
radiation,
conduction, and
convection. The usual oven temperature is below 300
°C, and radiation follows a
T4 law; so, radiative transfer of heat is inconsequential. Heat transfer is by conduction in the
air, and the more
efficienctt convection. More than a
century after
Joule's resistance heating
experiments, most kitchens have an additional type of oven, the
microwave oven. More than 90% of
United States households, my own included, have a microwave oven.
Most foods contain a lot of
water, and water is an excellent
absorber of
microwaves (see figure). Microwaves induce
rotation of
polar molecules, such as water, and the resulting
thermal energy heats the food in a process known as
dielectric heating. The
cavity magnetron of a microwave oven is likely the sole
vacuum tube now present in a house that typically contains billions of the
transistors that made the vacuum tubes in
consumer electronics obsolete. The
frequency of microwaves for cooking, 2.45
GHz, is in the middle of the
lowband Wi-Fi spectrum, 2.401 to 2.484 GHz.
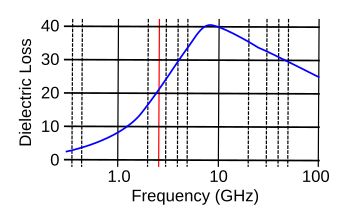
Dielectric loss of water at microwave frequencies. The 2.45 GHz frequency of microwave ovens is indicated by the red line.
(Data extracted from a Wikimedia Commons image. Click for larger image.)
Just as high-tech as a microwave oven is the
induction stovetop.
Induction heating of
pots and pans made of
cast iron or
ferromagnetic stainless steel is done by
alternating current in the frequency range between 25-50
kHz, just above
audio frequency. The low frequency
electromagnetic field induced by a
copper induction coil (see figure) causes generation of large
eddy currents in the cooking vessel to create resistive heating. Copper or
aluminum pots and pans won't heat by induction at these low frequencies, but a less efficient work-around is the placement of a
disk of a suitable
metal between the stovetop and the vessel.
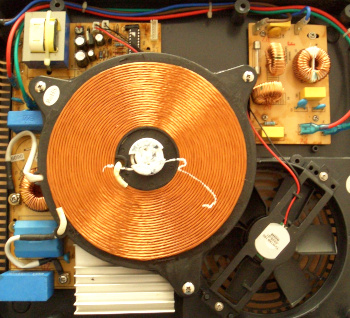
Large copper induction coil and associated electronics for induction cooking.
The coil is made from a heavy copper ribbon, since a kilowatt of power is typically required.
(Wikimedia Commons image by Walter Dvorak.)
There's physics in such kitchen devices, but there's also physics in food preparation as well. The June, 2023,
issue of
Physics of Fluids is a special issue on food physics. This issue contains an article about
fondant making by
physicists from the
Max Planck Institute for Polymer Research (Mainz, Germany), and the
Technische Universität Berlin (Berlin, Germany).[1-3] The article's content is freely available under a
Creative Commons Attribution License.
The scientific study of fondant making goes back at least a century with a 1919
publication by Mary Stephens Carrick in the
Journal of Physical Chemistry.[4] Carrick writes that fondant is made by
heating sugar in water and
acid, cooling this
syrup, and then
beating it vigorously.[4] Carrick writes that
cream of tartar (potassium acid tartrate) is preferred over
vinegar and
lemon juice as an acid, and that heating causes a portion of the sugar to be
inverted.[4] Relatively small changes in the amount of invert sugar have a marked effect on the
quality of the fondant.[4]
Fondant in the recent study was made by the
reduction of hot 80-92 wt-%
sucrose syrup, cooling the syrup while preventing preliminary
crystallization, and then
stirring the
supersaturated system.[1] This stirring causes rapid
nucleation and
crystal growth to form a
material in which
crystals make up between 40-75% wt-%, the percentage being dependent on the
composition and temperature.[1]
The crystals, which can be regular or irregular in shape, are ideally between 10-20
μm in
diameter.[1] The physics of fondant creation are difficult to
analyze, since the initial supersaturated solutions are
metastable, and crystallization happens under conditions that are far from
equilibrium.[1] Sucrose crystallization is inhibited by the low
mobility and
diffusivity of the sucrose
molecules.[1] The
kinetic energy of agitation, however, causes rapid nucleation and crystal growth.[1]
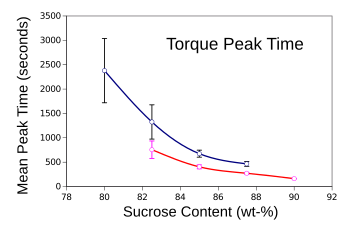
Fondant torque peak time as a function of sucrose content at 25 °C (top curve) and 44 °C (bottom curve).
The error bars are the standard deviation.
(Graphed using Gnumeric using data from the Supplementary Materials for Ref. 1.[2] Click for larger image.)
For proper experimental control, the research team used a
laboratory kneader; that, combined with
light microscopy allowed them to precisely observe the fondant creation process and develop
models using
parameters of agitation, temperature, and concentration.[1] Primary attention was paid to torque and its influence on crystallization.[1] Torque exhibited a characteristic
minimum followed by a sharp
peak during crystallization.[1] This effect is supposed to arise from an initial change in concentration of the
liquid phase, followed by the formation of large crystalline
conglomerates, and their eventual breakage.[1] Low torque indicates a smoother
texture, and the peak torque indicates a thicker solution that's characteristic of a fondant.[3]
Says
Thomas A. Vilgis, a
professor at the Max Planck Institute for Polymer Research and an
author of the paper,
"It was surprising to see the sugar crystals in the solution grow first during the early stages of stirring, and then the biggest get smaller again due to the stirring. Finally, their sizes adjust themselves, which leads to this fine creamy texture of fondants, provided the concentration, temperatures, and stirring speed are chosen correctly."[3]
Vilgis and co-author,
Ph.D. student Hannah Hartge intend to examine the behavior of fondants created with
sugar alternatives, such as
erythritol and
isomalt.[3]
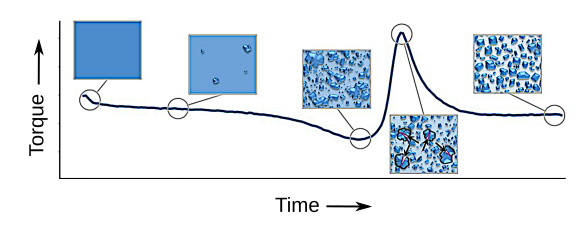
Torque and crystallization of fondant as a function of mixing time. The torque curve is the average during the kneading process of a solution with 85 wt-% sucrose. (Image by Hannah M. Hartge, enhanced to show detail.)
References:
- Hannah M. Hartge, Eckhard Flöter, and Thomas A. Vilgis, "Crystallization in highly supersaturated, agitated sucrose solutions, Physics of Fluids, vol. 35, no. 6 (June 27 2023), Article no. 064120, https://doi.org/10.1063/5.0150227.
- Supplementary Materials for Ref. 1 (zip file).
- Fondant: Where baking and thermodynamics mix, American Institute of Physics Press Release, June 27, 2023.
- Mary Stephens Carrick, "Some Studies in Fondant Making, J. Phys. Chem., vol. 23, no. 9 (December 1, 1919), pp. 589-602, https://doi.org/10.1021/j150198a001.
Linked Keywords: Connection; cooking; ; chemistry; chemist; cooks; chemical synthesis; ingredient; measurement; food preparation utensil; bowl; containment vessel; mixture; temperature; time; analytical chemistry; analytical chemist; sample (material); product (chemistry); chemical reaction; Maillard reaction; amino acid; flavor; food; browning (food process); bread; biscuit; French fries; pretzels; lye; Evan-Amos; Wikimedia Commons; bias; biased; physics; household; machine; mechanical device; can opener; blender; principle; refrigerator; Joule expansion; gas; cooling; thermodynamics; free expansion; technology; elastocaloric effect; magnetocaloric effect; resistor; resistance element; conventional oven; heat transfer; transfer of heat; thermal radiation; conduction (heat); convection; Celsius; °C; Stefan-Boltzmann law; T4 law; atmosphere of Earth; air; efficiency; efficienct; century; James Prescott Joule; experiment; microwave oven; United States; water; absorption (electromagnetic radiation); absorber; microwaves; rotation; molecular dipole; polar molecule; thermal energy; dielectric heating; cavity magnetron; vacuum tube; transistor; consumer electronics; frequency; hertz; GHz; lowband Wi-Fi spectrum; dielectric loss of water at microwave frequencies; induction cooking; cooktop; stovetop; induction heating; cookware and bakeware; pots and pans; cast iron; ferromagnetism; ferromagnetic; stainless steel; alternating current; kHz; audio frequency; electromagnetic field; electromagnetic induction; induce; copper; inductor; induction coil; eddy current; aluminum; disk (mathematics); metal; electronics; ribbon; kilowatt; electric power; Walter Dvorak; issue (periodical literature); Physics of Fluids; fondant; physicist; Max Planck Institute for Polymer Research (Mainz, Germany); Technische Universität Berlin (Berlin, Germany); Creative Commons Attribution License; scientific literature; publication; Journal of Physical Chemistry; heat; heating; sucrose; sugar; acid; syrup; agitator (device); beating; potassium bitartrate; cream of tartar (potassium acid tartrate); vinegar; lemon juice; inverted sugar syrup; quality control; reduction (cooking); crystallization; stirring; supersaturation; supersaturated; nucleation; crystal growth; material; crystal; chemical composition; micrometer; μm; diameter; analysis; analyze; metastability; metastable; thermodynamic equilibrium; mobility; diffusivity; molecule; kinetic energy; fondant torque peak time as a function of sucrose content; torque; maximum and minimum; peak; time; function (mathematics); error bar; standard deviation; Gnumeric; laboratory; kneader reactor; microscope; light microscopy; mathematical model; parameter; liquid; phase (matter); conglomerate; texture; Thomas A. Vilgis; professor; author; cream; creamy; Doctor of Philosophy; Ph.D.; postgraduate education; student; Hannah Hartge; sugar substitute; sugar alternative; erythritol; isomalt; mixing (process engineering); curve; average; aqueous solution.