Refining Germanium
June 22, 2017
One of my
brothers and I had the experience of working with Ernest ("Ernie") Buehler, he at
Bell Labs, and I at a
corporation whose name has been
expunged by a
merger and typical corporate
chessmanship. Ernie enjoyed
repairing small appliances for his
elderly lady friends, and he was
famous for his
potato bread. He was a
crystal grower, also famous for growing the
germanium crystal used in the
first transistor, invented at Bell Labs in December, 1947, by
John Bardeen (1908-1991),
William Shockley (1910-1989), and
Walter Brattain (1902-1987). This trio was awarded the 1956
Nobel Prize in Physics for this work. Buehler also grew the crystal for the first
silicon transistor.[1]

Commemorative plaque marking the demonstration of the transistor. This plaque was dedicated on December 8, 2009, during a ceremony at Bell Labs.
(My photo, via Wikimedia Commons. Click for larger image.)
While the
semiconductor of choice in today's world is silicon because of its better
temperature stability, germanium crystals are easier to grow, and this fact was especially important in the
1940s. Both silicon and germanium are prepared using the
Czochralski process in which a
seed crystal is dipped into a
crucible of
molten semiconductor just at the
melting point, and the seed grows larger when
rotated and pulled away at a slow rate. This method is used not just for semiconductors, but also for
laser materials such as
yttrium aluminum garnet (YAG, Y
3Al
5O
12).
The
Czochralski process is named after its
inventor,
Polish chemist,
Jan Czochralski. Czochralski wasn't interested in growing crystals; rather, he developed this process in 1916 in an attempt to produce
metal wire without
drawing.
Gordon Teal of Bell Labs adapted the process to the growth of germanium crystals that were used to fabricate
high frequency diodes during
World War II.
The first transistor wasn't a
junction transistor or
field effect transistor, both of which are now common. It was a
point-contact transistor, a type that was easy to demonstrate with the crude
laboratory equipment of the time. As shown in the figure, it was created by the contact of two closely-spaced
gold electrodes on a
slice of germanium crystal mounted on another electrode for
ohmic contact. The metal contacts form shallow
p-type regions in the overall
n-type germanium.
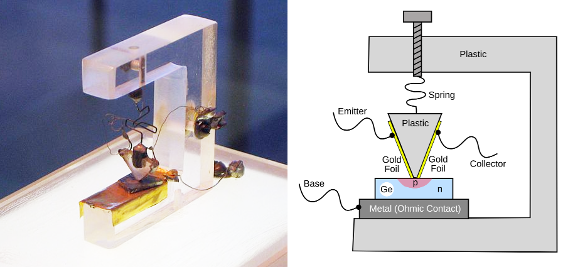
The first transistor was a point-contact transistor, improvised by Walter Brattain using a bent paper clip as a spring. In an amplifier circuit, a small current at the emitter generates a higher current between the base and collector. Once you have the germanium, the rest is relatively easy, a fact that shows the importance of materials science to progress in technology. (Left photo by Windell H. Oskay, via Wikimedia Commons; right image created using Inkscape. Another image of the first transistor can be found at archive.org)
When growing a crystal of germanium, or another
element or
compound, you need high
purity starting materials. The typical standard is "
five-nines;" that is, 99.999% pure, which is quite a step above
99 44/100% pure. Often, it's important that the 1000
ppm of impurities in such materials do not contain certain elements, such as
sodium.
Germanium is obtained as a
byproduct of
zinc refining from the chief
ore of zinc,
sphalerite, which is mostly
zinc sulfide (ZnS) with quite a few metal impurities substituting on the zinc
lattice sites in the crystal. One of these impurities is germanium, which generally exists at about 1000 ppm, but as high a
concentration as 3000 ppm in some ores.[2]
The
extraction process to separate germanium from zinc is not
environmentally friendly, since it uses
chlorine and
hydrochloric acid. Now, a team of
chemists from
McGill University (Montreal, Quebec, Canada), the
University of Western Ontario (London, Ontario, Canada), and
Soochow University (Suzhou, China) has developed an alternative process that uses a
recyclable quinone/
catechol redox reaction that uses
oxygen, rather than chlorine, as an
oxidizer to produce an air- and moisture-stable Ge(IV)-
catecholate that is converted to the high-purity germanium
organometallic compound,
germane.[3-4]
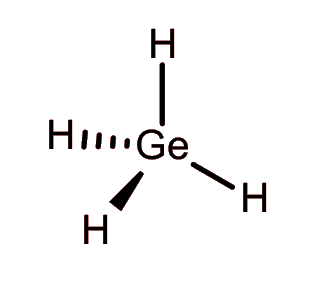
Germane.
This molecule is like methane, but with germanium as the central atom in place of carbon.
(Via Ref. 3.[3] Licensed under the Creative Commons Attribution-NonCommercial license.)
The inspiration for the process came from
biology. McGill University had been researching
melanin, the chemical that gives color to
skin and
hair and will also bind to metals.[4] The chemists decided to
synthesize a molecule that mimics such qualities of melanin to extract germanium at
room temperature without the use of
solvents.[4] Germanium is a good target for such research, since it's a useful element without a suitable substitute that exists as just a trace element in various ores.[3] Such compounds can be used to extract other metals, such as zinc,
copper,
manganese and
cobalt.[4]
Says
Jean-Philip Lumb, an
associate professor in the
McGill University Department of Chemistry.
"At a time when natural deposits of metals are on the decline, there is a great deal of interest in improving the efficiency of metal refinement and recycling, but few disruptive technologies are being put forth... That's what makes our advance so important... Applications of green chemistry lag far behind in the area of metals, yet metals are just as important for sustainability as any organic compound. For example, electronic devices require numerous metals to function."[4]
Their final process is green in more than one sense, since it also consumes far less energy than the conventional techniques.[3-4] One aspect of the process is the use of
mechanochemistry; that is, using
mechanical energy to promote
chemical reactions. The chemicals are shaken at high speed in
milling jars containing
stainless steel balls.[4-5]
Funding for this research was provided by the
Natural Sciences and Engineering Research Council of Canada, the
National Natural Science Foundation of China, and other agencies.
References:
- Oral-History:Goldey, Hittinger and Tanenbaum, Interview No. 480, IEEE History Center, The Institute of Electrical and Electronics Engineers, Engineering and Technology History Wiki, September 25, 2008.
- Nigel J. Cook, Barbara Etschmann, Cristiana L. Ciobanu, Kalotina Geraki, Daryl L. Howard, Timothy Williams, Nick Rae, Allan Pring, Guorong Chen, Bernt Johannessen, and Joël Brugger, "Distribution and Substitution Mechanism of Ge in a Ge-(Fe)-Bearing Sphalerite," Minerals, vol. 5 (March 24, 2015), pp. 117-132;, doi:10.3390/min5020117.
- Martin Glavinović, Michael Krause, Linju Yang, John A. McLeod, Lijia Liu, Kim M. Baines, Tomislav Friŝĉić, and Jean-Philip Lumb, "A chlorine-free protocol for processing germanium," Science Advances, vol. 3, no. 5 (May 5, 2017), Article e1700149, DOI: 10.1126/sciadv.1700149. This is an open access article with a PDF file available here.
- A more sustainable way to refine metals, McGill University Press Release, June 7, 2017.
- Solvent-free mechanochemical techniques..., YouTube Video by Michael Brand (University of Cardiff) and Jean-Louis Do (McGill University), June 6, 2017.
Permanent Link to this article
Linked Keywords: Brother; Bell Labs; corporation; expunge; merger; chessmanship; repair; small appliance; widow; elderly lady friend; famous; potato bread; crystal growth; crystal grower; germanium; crystal; first transistor; John Bardeen (1908-1991); William Shockley (1910-1989); Walter Brattain (1902-1987); Nobel Prize in Physics; silicon; transistor; commemorative plaque; semiconductor; temperature coefficient; temperature stability; 1940s; Czochralski process; seed crystal; crucible; melting; molten; melting point; rotation; rotate; laser; material; yttrium aluminum garnet; invention; inventor; Poland; Polish; chemist; Jan Czochralski; metal; wire; wire drawing; Gordon Teal; high frequency; diode; World War II; junction transistor; field effect transistor; point-contact transistor; laboratory equipment; gold; electrode; wafer; slice; electrical conductor; ohmic contact; p-type; n-type; paper clip; spring; amplifier; electrical network; circuit; electric current; emitter; base; collector; materials science; technology; evilmadscientist.com; Windell H. Oskay; Inkscape; archive.org; chemical element; chemical compound; impurity; purity; five-nines; Ivory (soap); 99 44/100% pure; parts-per notation; ppm; sodium; byproduct; zinc; extractive metallurgy; refining; ore; sphalerite; zinc sulfide (ZnS); crystal structure; lattice site; concentration; environmentally friendly; chlorine; hydrochloric acid; chemist; McGill University (Montreal, Quebec, Canada); University of Western Ontario (London, Ontario, Canada); Soochow University (Suzhou, China); recycling; recyclable; quinone; catechol; redox reaction; oxygen; oxide; oxidizer; catecholate; organometallic compound; germane; molecule; methane; atom; carbon; Creative Commons Attribution-NonCommercial license; biology; melanin; skin; hair; chemical synthesis; synthesize; room temperature; solvent; copper; manganese; cobalt; Jean-Philip Lumb; associate professor; efficiency; disruptive innovation; disruptive technologies; green chemistry; organic compound; mechanochemistry; mechanical energy; chemical reaction; milling jar; stainless steel; ball; funding; Natural Sciences and Engineering Research Council of Canada; National Natural Science Foundation of China.