Thermionic Solar Energy
August 24, 2010
The
photovoltaic cell is the device of choice for solar electric power in residences and commercial buildings. In such a cell, light is converted directly to electricity, and just a little circuitry is required to convert the generated voltages to the standard voltages needed to power toasters and televisions. The unfortunate thing about photovoltaic cells, aside from their expense, is their inability to utilize all the incident light energy. About half of the incident energy is wasted since it lies outside the
excitation band of the device. Since semiconductor photovoltaics function via electron excitations, there's a
quantum efficiency with which to contend. Much
R&D is applied to increasing this efficiency, typically through the introduction of new materials. The current material of interest is
copper-indium-gallium-selenide (CIGS), which is used in low cost thin film solar cells. The efficiency of cells of this material can be as high as about 20%. As I mentioned in a
previous article (Indium, January 8, 2008),
indium is becoming scarce, so alternatives are always welcome. These aren't the only problems. Photovoltaics are semiconductor materials, and semiconductor performance diminishes as the temperature increases.
One recently developed alternative is
Photon-enhanced thermionic emission (PETE), which uses both solar light and heat (Yes, solar heat is infrared light; but we're writing colloquially).
Thermionic emission was the driving force behind early electronics, since it's the principle used in
vacuum tubes.

My favorite vacuum tube, the 12AX7
Researchers at
Stanford University have developed a PETE solar energy harvester that works well at high temperatures.[1-3] Whereas
silicon cells fail to function above about 100
oC, the PETE devices start becoming effective above about 200
oC and work better at higher temperatures. This means that non-silicon materials, such as
gallium arsenide, are needed. The Stanford researchers used
gallium nitride[4] in their first devices. Another plus of high temperature operation is that waste heat not utilized by the PETE device can drive a conventional thermal engine to extract more power. PETE devices could be retrofitted onto existing solar thermal plants to generate electricity before transferring heat to the heat engine. The Stanford team, led by Assistant Professor
Nick Melosh, has projected a 55-60% efficiency for PETE devices, which is far in excess of that of present photovoltaics.
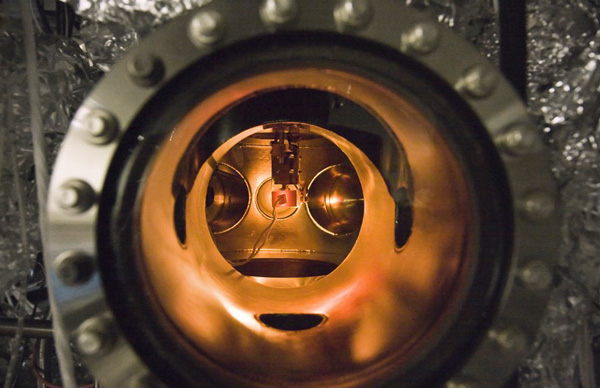
A small PETE device made with cesium-coated gallium nitride glows during testing inside an ultra-high vacuum chamber. The combination of a semiconductor substrate and a cesium layer is the key to device operation. (Photograph by Stanford University).
The PETE devices are intended to work with
solar concentrators, since just arraying them will not lead to the high temperatures needed for efficient operation. Use of a solar concentrator, such as a
parabolic reflector, has the added advantage that the PETE device itself can be very small, about 1% of the collection area. Says Melosh,
"...The material cost in this is not really an issue for us, unlike the way it is for large solar panels of silicon."[1]
In their experiments, the Stanford team showed that thermionic emission in a device without
insolation did not occur until about 1000
oC. This is the point at which electrons overcome the material
work function. High solar illumination of the same device caused the electron emission from the cathode at 350
oC to generate the same current that would normally require a temperature of 1350
oC for thermionic emission only.
The Stanford research was partially funded by the US
Department of Energy and the
Defense Advanced Research Projects Agency. It's published in a current issue of
Nature Materials.[3] Not surprisingly, a patent application has been filed. [5] Here's the first claim.
"Apparatus for radiant energy conversion, the apparatus comprising: a semiconductor photocathode having a positive electron affinity; and an anode separated from said photocathode; wherein absorption of incident radiation in said photocathode during operation of said apparatus gives rise to a distribution of electrons in a conduction band of said cathode; wherein some or all electrons in said distribution are thermalized with respect to a temperature of said photocathode, wherein said temperature of said photocathode is greater than 200oC. during operation of said apparatus; wherein some or all electrons in said distribution are emitted from said photocathode and received by said anode; wherein a potential difference is established between said photocathode and said anode by electrons received at said anode to provide output electrical power."
References:
- Louis Bergeron, "New solar energy conversion process discovered by Stanford engineers could revamp solar power production," Stanford University Press Release, August 2, 2010.
- Professor Nick Melosh of Stanford University describes how PETE devices work (non-technical explanation on YouTube).
- Jared W. Schwede, Igor Bargatin, Daniel C. Riley, Brian E. Hardin, Samuel J. Rosenthal, Yun Sun, Felix Schmitt, Piero Pianetta, Roger T. Howe, Zhi-Xun Shen and Nicholas A. Melosh, "Photon-enhanced thermionic emission for solar concentrator systems," Nature Materials, (Published online August, 1, 2010).
- "Gallium Nitride Crystals," This Blog, July 29, 2010,
- Jared Schwede, Nicholas Melosh and Zhixun Shen, "Photon Enhanced Thermionic Emission," United States Patent Application 20100139771 (October 16, 2009)
Permanent Link to this article
Linked Keywords: photovoltaic cell; excitation band; quantum efficiency; R&D; copper-indium-gallium-selenide; indium; Photon-enhanced thermionic emission; Thermionic emission; vacuum tubes; 12AX7; Stanford University; silicon; gallium arsenide; gallium nitride; Nick Melosh; solar concentrators; parabolic reflector; insolation; work function; Department of Energy; Defense Advanced Research Projects Agency; DARPA; Nature Materials.