Bridgmanite
March 7, 2022
Among the
artifacts of
20th century corporate research laboratories are
ornate analytical balances,
workaday triple beam balances, and less impressive
journal routing slips. These routing slips were lists of names and associated mail drop locations that were attached to recent journal issues to allow their distribution to laboratory members. After receiving the journal issue in your
mail slot, you had the obligation to quickly scan its
contents, cross your name from the list, and place it in the outgoing mail. The corporate
library had
an arrangement in which they could make you a
legal copy of any
paper that caught your interest. Routing slips were another item quickly displaced by the
Internet, as were many members of the library
staff.
I was on the routing list for
New Scientist, a
magazine with
science and
technology news, and articles of a more
popular than
ponderous nature. New Scientist also had some
reader feedback columns, the feedback in those days done by
regular mail and not
email. One column that I enjoyed reading posed questions to the readership about using science to solve everyday problems. I enjoyed creating my own solutions to these questions and reading the submitted answers of those who were willing to devote enough effort to create a
letter and pay for a
stamp.
One question was as follows. You have two
geometrically equivalent
spoons, one of which is
sterling silver (an
alloy that's 92.5%
silver and 7.5%
copper), and the other is the less valuable
silver-plated stainless steel. How can you distinguish them? One obvious way is by
weighing them, since sterling silver is about 10% more
dense than stainless steel. Since most
homes don't have laboratory balances, I decided on a different solution. Since the
thermal conductivity of sterling silver is more than ten times that of stainless steel, you would dip them both into
boiling water, and the one you would drop first would be the sterling silver spoon.
As we all know, we sit on
Earth's surface at the top of a very
hot ball of
solid and
liquid materials, but it's the relatively low thermal conductivity of intermediate layers that keeps Earth's surface near our desired
room temperature. The
Earth's inner core is as hot as 7000
K, but it's at a
pressure great enough that it exists as a solid ball of
iron-nickel alloy. Just above the inner core is the
outer core of liquid
iron and
nickel at
temperatures from 3000 K - 5000 K. Above this are the upper and lower
mantle of
silicate minerals that comprise about two-thirds of the
mass of the
Earth existing from 1750 K - 3000 K. (see figure)
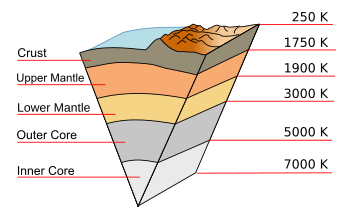
A cutaway diagram of the Earth along with temperatures along the geothermal gradient.
This diagram (except for the mountain surface features) is to scale.
(Modified Wikimedia Commons image. Click for larger image.)
The
silicates that
insulate us from the inner Earth are
silicate perovskite ((Mg,Fe)SiO3), also known as
bridgmanite, and
calcium silicate perovskite (CaSiO3), also known as
davemaoite, and
magnesiowüstite ((Mg,Fe)O). Davemaoite is named after
geophysicist,
Ho-kwang (Dave) Mao (b. 1941), and bridgmanite is named after 1946
Nobel Physics Laureate,
Percy Bridgman (1882-1961), who was a
pioneer in
high pressure research and an originator of the
Bridgman-Stockbarger method of
crystal growth. Bridgman was able to achieve pressures in his laboratory exceeding 10
GPa (100,000
atmospheres) that allowed the study of the
physical properties of many high pressure
minerals.
Bridgmanite exists only at high-pressures, and it occurs at about 660
kilometers deep within the Earth where the pressure is about 24 GPa. Bridgmanite becomes
unstable at higher pressures, at a depth of about 2700 kilometers. Bridgmanite constitutes around 80% of the mantle. with
calcium perovskite at about 10%
concentration. It is formed in the laboratory using
laser heating in
diamond anvil cells.
Perovskite-structured (Mg,Fe)SiO
3 was named bridgmanite by the
Commission on New Minerals, Nomenclature and Classification of the
International Mineralogical Association in 2014.
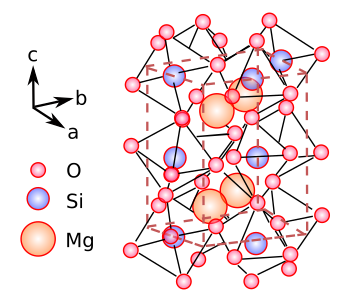
Crystal structure of bridgmanite, shown as MgSiO3.
Bridgmanite has the orthorhombic crystal structure (Space group, Pnma). It has silicon atoms surrounded by oxygen in six-fold coordination.
It's evident that only very high pressures can allow the large magnesium cations to exist in such close proximity to each other.
(Created using Inkscape from original image in ref. 1.[1] Click for larger image.)
As can be expected, the thermal conductivity of bridgmanite greatly affects the
cooling rate of Earth's core. Recently, an international
research team from
ETH Zürich, (Zürich, Switzerland), the
Carnegie Institution of Washington (Washington, DC, USA), the
University of Bayreuth (Bayreuth, Germany), and
Okayama University (Tottori, Japan) has used a recently developed
optical absorption measurement system in a
pulsed laser heated diamond anvil cell to measure the thermal conductivity of bridgmanite.[2-3] They found that the thermal conductivity of bridgmanite is about 1.5 times higher than assumed previously.[2-3] Their research is published in an
open access paper in a recent issue of
Earth and Planetary Science Letters.[2]
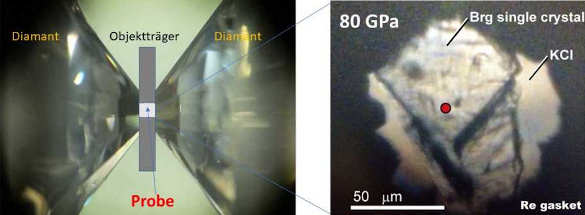
Bridgmanite in a laser-heated diamond anvil cell for thermal conductivity measurement. (ETH-Zürich image by Murakami M, et al.)
As the study
authors write, the
radiative thermal conductivity of bridgmanite had not been accurately measured.[2] Their measurement at 80 GPa showed that the radiative thermal conductivity of bridgmanite at the core-mantle boundary should be about 5.3±1.2
W/
m-
K.[2] The more rapid cooling of the mantle will result in a
positive feedback process in which bridgmanite turns into the mineral,
post-perovskite (another
phase of MgSiO
3), and the post-perovskite will accelerate cooling even further, since this mineral is a better thermal conductor than bridgmanite.[2-3] This more efficient cooling of the mantle will ultimately weaken many
tectonic activities.[2] As study author and ETH
professor,
Motohiko Murakami, summarizes,
"Our results... suggest that Earth, like the other rocky planets, Mercury and Mars, is cooling and becoming inactive much faster than expected."[3]
References:
- What’s in a name? – Bridgmanite, Blogging a Crystal Structure a day in 2014 at the Crystallography365 website, July 8, 2014.
- Motohiko Murakami, Alexander F. Goncharov, Nobuyoshi Miyajima, Daisuke Yamazaki, and Nicholas Holtgrewe, "Radiative thermal conductivity of single-crystal bridgmanite at the core-mantle boundary with implications for thermal evolution of the Earth," Earth and Planetary Science Letters, vol. 578, Article no. 117329, January 15, 2022, https://doi.org/10.1016/j.epsl.2021.117329. This is an open access article under a Creative Commons license.
- Peter Rüegg, "Earth's interior is cooling faster than expected, ETH-Zürich Press Release, January 14, 2022.
Linked Keywords: Cultural artifact; 20th century; research and development; corporate research; laboratory; ornate; analytical balance; workaday; triple beam balance; scientific journal; mailing list; routing slip; letter box; mail slot; table of contents; library; Copyright Clearance Center; law; legal; scientific literature; paper; Internet; employment; staff; New Scientist; magazine; science; technology; news; popular culture; ponderous; reader; feedback; column (periodical); regular mail; email; letter (message); postage stamp; geometry; geometrically; spoon; sterling silver; alloy; silver; copper; silver plating; silver-plated; stainless steel; weighing; density; dense; home; thermal conductivity; boiling; water; lithosphere; Earth's surface; heat; hot; sphere; ball; solid; liquid; material; room temperature; Earth's inner core; kelvin; pressure; iron-nickel alloy; Earth's outer core; iron; nickel; temperature; mantle (geology); silicate mineral; mass; Earth; Earth cutaway with temperatures; cutaway diagram; geothermal gradient; mountain; topography; surface feature; linear scale; Wikimedia Commons; silicate mineral; thermal insulation; insulate; silicate perovskite ((Mg,Fe)SiO3); bridgmanite; calcium silicate perovskite (CaSiO3); davemaoite; ferropericlase; magnesiowüstite ((Mg,Fe)O); geophysics; geophysicist; Ho-kwang (Dave) Mao (b. 1941); Nobel Prize in Physics; Nobel Physics Laureate; Percy Bridgman (1882-1961); innovator; pioneer; high pressure research; Bridgman-Stockbarger method; crystal growth; pascal (unit); GPa; atmosphere (unit); physical property; mineral; kilometer; chemical stability; calcium perovskite; concentration; laser; heater; heating; diamond anvil cell; perovskite structure; Commission on New Minerals, Nomenclature and Classification; International Mineralogical Association; crystal structure of bridgmanite; orthorhombic crystal system; orthorhombic crystal structure; space group; silicon; atom; oxygen; coordination number; six-fold coordination; high pressure; magnesium; cation; Inkscape; cooling; rate (mathematics); research; ETH Zürich, (Zürich, Switzerland); Carnegie Institution of Washington (Washington, DC, USA); University of Bayreuth (Bayreuth, Germany); Okayama University (Tottori, Japan); absorption (electromagnetic radiation); optical absorption; measuring instrument; measurement system; pulsed laser; open access paper; Earth and Planetary Science Letters; laser; heater; heated; diamond anvil cell; author; radiative cooling; watt; meter; kelvin; positive feedback; post-perovskite; phase diagram; phase; tectonics; tectonic activity; professor; Motohiko Murakami; terrestrial planet">rocky planet; Mercury (planet); Mars.