Boiling Crisis
May 20, 2019
Bubbles might be something that you rarely think about, but they're ubiquitous in your life. When you arise in the morning, there are bubbles in your
toothpaste,
mouthwash,
soap,
shampoo,
shaving cream,
boiling water for your
oatmeal, and
foam atop your
coffee. If you're a hearty
breakfast person, there are bubbles in your
bacon grease as it
fries. There are other examples throughout your
working day, and likely many more on
weekends.

A famous literary reference to bubbles is found in Shakespeare's Macbeth (c.1605), where the Three Witches are tending their witches' brew and chanting, "Double, double toil and trouble; Fire burn and caldron bubble."
English allowed multiple spellings in Shakespeare's time, and some are carried over today, when caldron is also spelled as cauldron.
(Portion of a 19th century woodcut, image 26345-V0025894 from the Wellcome Trust, via Wikimedia Commons. Click for larger image.)
Greek philosopher,
Aristotle (384-322 BC) had a simple explanation for the observation that bubbles rise in a
liquid. In
Aristotle's version of physics, things had a tendency to move to their
natural place. In the case of the
air in a bubble, the natural place is above the water. The
four elements of classical antiquity were arrayed in successive layers above the center of the universe, seen at that time as the center of the Earth, with
Earth being the lowest, layered above by
Water,
Air and
Fire.
Bubbles are usually observed when a liquid is
heated beyond its
boiling point and its
vapors coalesce inside it. There is, however, another way to create bubbles in a liquid, and that's by a
mechanical action or
flow condition that causes a rapid change in
pressure and produces small bubbles where the pressure is relatively low. This would be merely a
scientific curiosity were it not for
shock waves that are produced when these bubbles collapse.
This
phenomenon is called
cavitation, and it's responsible for damage to many types of
machines operating in fluids. As I wrote in a
previous article (Cavitation, October 16, 2017), cavitation causes damage to
ship propeller blades,
hydraulic pump impellers, and
pipe bends that cause sudden change in the direction of rapidly moving liquid.
The term, "cavitation," was coined by R. E. Froude and first used by Barnaby and Thornycroft in 1893.[1] Barnaby and Parsons determined that the
propeller failure of the
HMS Daring, a
British warship was caused by cavitation. Although not by name, cavitation was earlier described
Leonhard Euler (1707-1783) in his 1754 paper, "Théorie plus complette des machines qui sont mises en mouvement par la réaction de l'eau (A more complete theory of machines that are set in motion by reaction with water).[2]
The cavitation phenomenon was of interest to another
physics luminary after Euler.
Rayleigh analyzed the collapse of a void in a large liquid mass in 1917,[3] and the
Rayleigh–Plesset equation describes the
dynamics of cavitation using the simplifying assumption that the void is a
sphere. Actual voids are more
hemispherical in shape, since they need to be
nucleated on a
surface.

The first lesson learned by any cook is how to boil water.
While addition of salt does cause the boiling temperature to increase, the effect is so small that the temperature change for an amount added in cooking (as for cooking pasta) would hardly register on a thermometer.
(Photograph via Wikimedia Commons)
Bubbles can be created by other means than the mechanical action in cavitation.
Transient local
heating in a liquid by a
focused laser pulse or an
electrical discharge, will create a bubble. The
pressure increase in a bubble during collapse will increase the
temperature of the bubble
gases, sometimes to thousands of degrees
kelvin, and this can result in an effect called
sonoluminescence in which
light is emitted from the bubble.
Liquids can sometimes be put into a
superheated state in which the boiling temperature is exceeded, but boiling does not occur. That happens in
smooth containers for which there are no
nucleation sites for bubbles. To prevent superheating and ensure streams of small bubbles rather than large bubbles that might eject liquid from
beakers,
chemists add
boiling stones to their beakers. These small, rough, irregularly-shaped
ceramic pieces provide nucleation sites for bubbles.
The nucleation of bubbles at
surfaces is an important factor in the
transport of heat from solids to liquids, since the
thermal conductivity of
gases is usually lower than that of liquids. The low thermal conductivity of air is the principle behind
insulating foams that contain air pockets, and an insulating layer of vapor is responsible for the longevity of water
droplets placed in a very hot
pan first noticed by
German physician,
Johann Gottlob Leidenfrost in 1756.
The
eponymous Leidenfrost effect occurs when a thin
vapor layer is formed below a droplet placed on a surface that's above the liquid's boiling point. The vapor barrier offers thermal insulation, the liquid can't extract
heat from the pan at a rapid rate, so its
evaporation is inhibited.
While the Leidenfrost effect is a nice
party trick, a vapor layer can cause a problem in
heat exchangers. This is especially true in a regime called a
boiling crisis, which is the point at which so many bubbles are nucleated on a hot surface that they coalesce into a continuous sheet of vapor. This vapor sheet seriously inhibits heat transfer to the liquid, thereby counteracting the purpose of the heat exchanger. For this reason, such devices as
nuclear reactors are operated only in a safe regime far below the
temperatures that could trigger a boiling crisis.
A boiling crisis is not a problem for your
electric coffee maker, unless you like your
coffee really hot, since the phenomenon only happens for water at one
atmosphere pressure when the
heat flux across the solid-liquid interface nears a
megawatt per
square meter. The heat flux at which a boiling crisis occurs at a
horizontal surface can be estimated for large objects with very small surface
curvature from the following
equation, derived by Novak Zuber in 1959:[4]
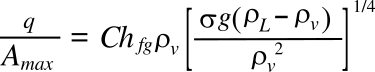
In this equation,
q is the
critical heat flux,
L is the
latent heat of vaporization,
ρL and
ρv are the
densities of the liquid and vapor,
σ is the
surface tension, and
g is the
gravitational acceleration. It's interesting to note from this equation that the critical heat flux is zero in a
weightless environment, where
g = 0.
This boiling crisis is important to
nuclear power plants, which must exchange large quantities of heat in order to drive
turbines for
electrical power generation. Since a boiling crisis would lead to a
runaway weakening or
melting of
material, nuclear plants are designed to operate at
safe levels that are far below boiling crisis conditions. A team of
scientists and
engineers from the
Department of Nuclear Science and Engineering, the
Massachusetts Institute of Technology (Cambridge, Massachusetts) has recently investigated the boiling crisis in detail and has published their findings in a recent issue of
Physical Review Letters.[5-6]
Proper heat exchange in nuclear reactors is important, since it prevents overheating that could potentially lead to a
meltdown.[6] For safety reasons,
regulations require that nuclear plants operate at heat fluxes that are no more than 75 percent of the critical heat flux, the heat flux for a boiling crisis.[6] Since the boiling crisis process is not well understood, the critical heat flux is calculated
conservatively, so the
power produced by a nuclear reactor is less than it might be.[6]
The MIT team is led by
assistant professor,
Matteo Bucci, and it includes
graduate students Limiao Zhang and
Jee Hyun Seong.[6] This
research has application beyond nuclear reactors, since effective heat transfer is required in other types of power plants, and also for liquid cooling of
high-performance computer chips.[6] Says Bucci
"Even in the 21st century, we talk about an energy revolution, a computer revolution, nanoscale transistors, all kinds of great things. Yet, still in this century, and maybe even in the next century, these are all limited by heat transfer."[6]
The critical heat flux equation written above was derived using some
simplifying assumptions. In actual cases, small changes in material types and
surface texture can have large affects, as evidenced when
limescale disrupts
water heater performance. The MIT team
modeled the boiling crisis by taking such factors into account, and they found that the phenomenon is much like the
flow of traffic in a
city, or how
disease epidemics spread.[6]
In the traffic case, increasing the number of
cars beyond a certain
threshold results in a greater
likelihood that they will bunch up into a
traffic jam. The bubbles on a heated surface show a similar behavior in which bubbles will crowd together and merge when a critical density is reached.[6]
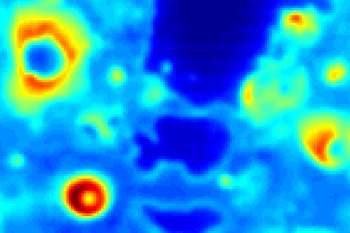
A color map of the rate of heat transfer from a metal surface, with red the highest and blue the lowest.
The large blue areas show the beginning of a boiling crisis.
(MIT image by Zhang, Seong, and Bucci.[5])
Says Bucci, "The boiling crisis is essentially the result of an accumulation of bubbles that merge and coalesce with each other, which leads to failure of the surface... We can take inspiration, take the same approach to model boiling as is used to model traffic jams."[6] Fortunately, such traffic models are well developed.[6] Modifying the surface texture to minimize the interaction between bubbles is one way to prevent the boiling crisis at a certain condition.[6] This might allow an increase the critical heat flux by 10-20 percent and allow better
fuel efficiency in power plants.[6]
References:
- Shengcai Li, Christopher E. Brennen, and Yoichiro Matsumoto, "Introduction for amazing (cavitation) bubbles," Interface Focus, vol. 5, no. 5 (October 6, 2015), DOI: 10.1098/rsfs.2015.0059.
- L. Euler, "Théorie plus complette des machines qui sont mises en mouvement par la réaction de l'eau," (A more complete theory of machines that are set in motion by reaction with water), Mémoires de l'Académie Royale des Sciences et des Belles Lettres à Berlin, vol. 10 (1754), pp. 227-295 (via Google Books).
- Lord Rayleigh, "On the pressure developed in a liquid during the collapse of a spherical cavity," Phil. Mag., vol. 34, no. 200 (1917), pp. 94-98, doi:10.1080/14786440808635681.
- Novak Zuber, "Hydrodynamic Aspects Of Boiling Heat Transfer," Thesis-University of California, Los Angeles, June 1, 1959, doi:10.2172/4175511 (PDF File).
- Limiao Zhang, Jee Hyun Seong, and Matteo Bucci, "Percolative Scale-Free Behavior in the Boiling Crisis," Phys. Rev. Lett., vol. 122, no. 13 (April 5, 2019), Article No. 134501, DOI:https://doi.org/10.1103/PhysRevLett.122.134501. Also available at arXiv.
- David L. Chandler, "Getting to the bottom of the 'boiling crisis'," MIT Press Release, April 4, 2019.
Linked Keywords: Bubble; toothpaste; mouthwash; soap; shampoo; shaving cream; boiling; water; oatmeal; foam; coffee; breakfast; bacon fat; bacon grease; frying; fry; working day; weekend; Wellcome Trust; literature; literary; William Shakespeare; Macbeth (c.1605); Three Witches; potion; witches' brew; chant; chanting; English language; spelling; caldron; cauldron; Wikimedia Commons; Ancient Greek philosophy; Greek philosopher; Aristotle (384-322 BC); liquid; Aristotelian physics; Aristotle's version of physics; natural place; atmosphere of Earth; air; four elements of classical antiquity; Earth (classical element); Water (classical element); Air (classical element); Fire (classical element); heat; heated; boiling point; vapor; coalescence (physics); coalesce; mechanical; fluid dynamics; flow; pressure; science; scientific; curiosity; shock wave; phenomenon; cavitation; machine; ship propeller blade; hydraulic pump; impeller; pipe (fluid conveyance); HMS Daring (1893); Great Britain; British; warship; Leonhard Euler (1707-1783); physics; John William Strutt, 3rd Baron Rayleigh; Rayleigh–Plesset equation; dynamics (mechanics_; sphere; hemisphere; nucleation; nucleated; surface; lesson; cook; iodised salt; boiling-point elevation; pasta; thermometer; transient state; heat; heating; focus (optics); focused; laser; pulse; electrical discharge; pressure; temperature; gas; kelvin; sonoluminescence; light; superheating; superheated; surface roughness; smooth; container; beaker (glassware); chemist; boiling chip; boiling stone; ceramic; surface; transport phenomenon; thermal conductivity; insulating foam; droplet; frying pan; Germany; German; physician; Johann Gottlob Leidenfrost; eponymous; Leidenfrost effect; vapor; evaporation; party trick; heat exchanger; boiling crisis; nuclear reactor; electric coffee maker; coffee; atmosphere (pressure); heat flux; megawatt; square meter; horizontal; curvature; equation; critical heat flux; enthalpy of vaporization; latent heat of vaporization; density; surface tension; gravitational acceleration; weightlessness; weightless environment; nuclear power plant; steam turbine; electrical power generation; positive feedback; runaway; melting; material; safety factor; safe level; engineer; Department of Nuclear Science and Engineering; Massachusetts Institute of Technology (Cambridge, Massachusetts); Physical Review Letters; nuclear meltdown; regulation; conservative; power; assistant professor; Matteo Bucci; postgraduate education; graduate student; Limiao Zhang; Jee Hyun Seong; research; supercomputer; high-performance computer chip; 21st century; energy; revolution; computer; nanoscopic scale; nanoscale; transistor; spherical cow; simplifying assumption; surface finish; surface texture; limescale; water heater; computational model; modeled; traffic flow; flow of traffic; city; disease epidemic; automobile; car; threshold; likelihood; traffic congestion; traffic jam; false color; color map; rate (mathematics); heat transfer; metal; fuel efficiency.