Transparent Amorphous Oxide
January 29, 2018
Fake news is not a recent
phenomenon. From
childhood, I've seen many impossible stories in the
tabloids arrayed at
supermarket checkouts. Also, several
alternative facts were a part of my childhood
science education. One of these was the idea that
glass was a
liquid that continued to
flow long after it cooled to
room temperature.
Glass is not a
crystalline solid as are most of the
inorganic materials that we encounter daily. Examples of these crystalline solids are
metals and
table salt. The
atoms in
crystals are regularly arrayed in a
lattice structure, while the atoms in glass are
randomly located, in
analogy to the atoms in a liquid. So, a glass must be a liquid, right? The cited "proof" of glass being a liquid is that the
stained glass panels in
centuries-old cathedrals are thicker at the bottom, so the glass flowed in response to
gravity just as a liquid would.

Glassmaking started before 3000 BC. As noted in Pliny's Natural History, both furnace technology and glassmaking were extensively developed in the 1st century AD
An image of Hasdrubal Aufdinger, the glaser, from the house book of Landauer Zwölfbrüderstiftung, vol. 1 (Nuremberg 1511-1706).
(Collection of the City Library Nuremberg, via Wikimedia Commons)
As it turns out, the varied thickness of the glass is just an
artifact of how the glass was
manufactured. The
artisans just arranged the thicker part at the bottom by artistic
convention. As explained on the
website of the
Corning Glass Museum, the
calculated flow of glass is
infinitesimally small.[1] I visited the Corning Glass Museum, which is a three hour's drive from my home town, when I was in
high school. This was perhaps one
catalyst for my
career in
materials science.
So, where do we place the
neither fish nor fowl, neither crystalline solid nor liquid, material called glass? Glass is an
amorphous solid, a solid that's a frozen image of a liquid. Metals can be produced as glasses, aptly named
metallic glass. One
commercially important metallic glass was developed by
AlliedSignal (now,
Honeywell) and is
trademarked as
Metglas®. Metallic glasses have
magnetic properties that make them suitable for
transformer cores and
magnetic shielding (see photo).

Metglas® transformer cores.
These cores are the SA1 composition with boron (1-5%), iron (85-95%), and silicon (5-10%).[2]
(photo by the author.)
Metallic glasses closely resemble metals, so they don't possess the
transparency that makes most glass valuable. There's a wide range of transparent glass compositions, each developed to serve a particular function. These functions can be as simple as
window glazing, or as technically advanced as
conductive glass for
computer and
cellphone displays. The following table gives a few examples of transparent glass compositions.
While an amorphous, glassy, structure can be locked-in at room temperature,
heating of amorphous materials allows the movement of atoms and attendant
crystallization. In the case of metallic glasses, heating to high temperatures allows the formation of
nanoscale crystallites in the amorphous
matrix that affect
material properties such as
heat capacity,
electrical conductivity and
magnetism. Magnetic metallic glasses have typically low
coercivity, but creation of nanocrystallites can increase coercivity substantially.
Crystallization will convert an originally transparent amorphous polymer to a cloudy,
translucent material.
Integrated optical devices need to be made from transparent materials that resist changes in their properties during high
temperature processing. Such thermal treatment can be required to
deposit or
anneal a material in an integrated optical device, causing glassy transparent oxides to crystallize and ruin their transparency. To overcome this problem, a team of
engineers and
materials scientists from the
Toyohashi University of Technology (Toyohashi, Aichi, Japan), the
Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology (Saitama, Japan), and the
Massachusetts Institute of Technology (Cambridge, Massachusetts) has investigated a high
refractive index thermally-stable amorphous
tantalum-
yttrium oxide with low
infrared absorption for use in
magneto-optical devices.[3-4]
The amorphous tantalum yttrium oxide is formed by the addition of a small amount of
yttrium oxide (Y
2O
3) to
tantalum oxide (Ta
2O
5). Tantalum oxide is widely used as a high refractive index material since it's a thermally stable oxide with high optical transmissivity.[3] While tantalum oxide has a 300
°C higher crystallization and glass-transition temperature than other high refractive index oxides such as
titanium oxide (TiO
2) and
hafnium oxide (HfO
2), it's still not stable at the 800 °C
oxygen anneal required for magneto-optical
garnet crystallization. It's only after such crystallization that these garnets exhibit high
Faraday rotation.[3]
Crystallization is sometimes suppressed by adding
elements of a smaller
ionic radius to a host material. For example, the crystallization temperature of HfO
2 is increased when La
2O
3 is added,
lanthanum being larger than
hafnium.[3] To suppress the crystallization of Ta
2O
5, the larger yttrium ion in the form of Y
2O
3 can be added.[3] This was the approach taken in the current study with the addition of about 14% Y
2O
3, which was enough to increase the crystallization temperature to about 850°C.[4]
Toyohashi University of Technology Ph.D. candidate, Takuya Yoshimoto, holding a film of amorphous tantalum-yttrium oxide.
Image copyright © Toyohashi University of Technology, all rights reserved.
Magnetron sputtering was used to fabricate layers of the high refractive index amorphous yttrium-tantalum oxide as part of a
dielectric mirror used in an iron garnet magneto-optical device operating at
near-infrared wavelengths.[3] The magnetic garnet required thermal treatment at about 750°C.[4] Aside from magneto-optical devices, the tantalum-yttrium oxide material would be useful for
spintronic,
magnonic, and
multiferroic devices as well.[3] Says
Taichi Goto, an
assistant professor at the Toyohashi University of Technology,
"We used amorphous tantalum yttrium oxide to form a dielectric mirror and combined it with magnetic garnet. Actually, other than magnetic garnet, there are more materials that have not been combined with dielectric mirrors because of the high-temperature thermal treatment that is required. I hope that our findings will help make such materials usable too."[4]
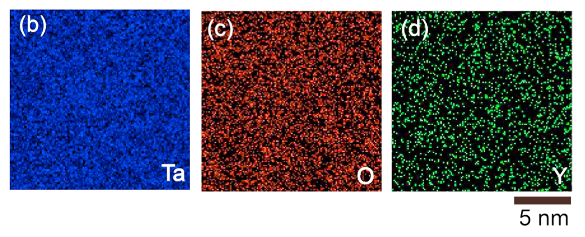
Element maps of tantalum, yttrium, and oxygen in an amorphous Ta-Y-oxide. These maps, created using the EDX technique, demonstrate the amorphous nature of the oxide. (Portion of fig. 5 of Ref. 3, licensed under the Creative Commons Attribution 4.0 International License.)
References:
- Robert Brill, "Does Glass Flow?" The Corning Museum of Glass Web Site, September 29, 2011.
- Material Safety Data Sheet, METGLAS® 2605 SA1 Iron Based Alloy, Metglas Inc. Web Site (PDF File).
- Takuya Yoshimoto, Taichi Goto, Hiroyuki Takagi, Yuchi Nakamura, Hironaga Uchida, Caroline A. Ross, and Mitsuteru Inoue, "Thermally stable amorphous tantalum yttrium oxide with low IR absorption for magnetophotonic devices," Scientific Reports, vol. 7, Article no. 13805 (October 23, 2017), doi:10.1038/s41598-017-14184-4. This is an open-access paper with a PDF file here.
- High-refractive-index material retains high transmissivity after annealing at 850 degrees C, Toyohashi University of Technology Press Release, November 27, 2017.
Linked Keywords: Fake news; phenomenon; childhood; tabloid; supermarket; point of sale; checkout; alternative facts; science; education; glass; liquid; fluid dynamics; flow; room temperature; crystal; crystalline; solid; inorganic compound; inorganic material; metal; table salt; atom; crystal structure; lattice; randomness; random; analogy; stained glass; century; centuries-old; cathedral; gravitation; gravity; history of glass; Roman glass; Pliny the Elder; Natural History (Pliny); furnace; technology; glassmaking; 1st century AD; Wikimedia Commons; artifact; manufacturing; manufactured; artisan; convention; website; Corning Glass Museum; calculation; calculated; infinitesimal; infinitesimally; high school; catalysis; catalyst; career; materials science; neither fish nor fowl; amorphous solid; amorphous metal; metallic glass; commerce; commercial; AlliedSignal; Honeywell; trademark; Metglas; magnetic; transformer; magnetic core; electromagnetic shielding; boron; iron; silicon; transparency; window glazing; electrical conductor; conductive; computer; mobile phone; cellphone; display; soda-lime glass; silicon dioxide; SiO2; sodium oxide; Na2O; calcium oxide; CaO; aluminum oxide; Al2O3; bottle; flint glass; lead(II) oxide; PbO; barium oxide; BaO; lens; crown glass; borosilicate glass; boron trioxide; B2O3; laboratory glassware; labware; fused quartz; indium tin oxide; ITO; indium(III) oxide; In2O3; tin dioxide; SnO2; computer monitor; heat; heating; crystallization; nanoscopic scale; nanoscale; composite material; matrix; material properties; heat capacity; electrical conductivity; magnetism; coercivity; translucent; photonic integrated circuit; Integrated optical device; temperature; planar processing; physical vapor deposition; annealing; anneal; engineer; materials scientist; Toyohashi University of Technology (Toyohashi, Aichi, Japan); Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology (Saitama, Japan); Massachusetts Institute of Technology (Cambridge, Massachusetts); refractive index; tantalum; yttrium; oxide; infrared; absorption (electromagnetic radiation); magneto-optic effect; magneto-optical; yttrium oxide; tantalum oxide; Celsius; °C; titanium oxide; hafnium oxide; oxygen; garnet; Faraday effect; Faraday rotation; chemical element; ionic radius; lanthanum; hafnium; Doctor of Philosophy; Ph.D. candidate; coating; film; amorphous; sputter deposition; magnetron sputtering; dielectric mirror; near-infrared wavelengths; spintronic; magnonic; multiferroic; Taichi Goto; assistant professor; map; energy-dispersive X-ray spectroscopy; EDX technique; Creative Commons Attribution 4.0 International License.