Nanowire Ink
January 30, 2017
The
materials used in today's
physics laboratory are quite different from those of the past.
Ernest Rutherford (1871-1937), a
physicist who was awarded the 1908
Nobel Prize in Chemistry, was famous for building
experiments from simple items such a piece of a
bicycle handlebar,
string, and
sealing wax.[1]
Natural leather was often used as a
gasket material, since it remains pliable at
low temperatures.

Ernest Rutherford (1871-1937).
Rutherford is famous for the discovery of the proton, but he was also Director of the Cavendish Laboratory of Cambridge University when James Chadwick discovered the neutron in 1932.
(Wikimedia Commons image, modified for artistic effect.)
Aquadag was another common material in physics laboratories of yesteryear. Aquadag, a
colloid of
water and
graphite is applied like a
paint, and it's a reasonable
conductor of electricity when it dries.
Solutions diluted 1:1 with water produce coatings with a
sheet resistance of about 800
ohms/square when air dried, but this decreases to just 20-30 ohms/square after heating at 300
°C.[2] For comparison,
copper has a sheet resistance of less than a milliohm per square.
The main
industrial application of aquadag was as an
electrode material for
cathode ray tubes where the high resistance isn't a problem since the
currents are low. Since graphite is a good
dry lubricant, aquadag and a variant containing
molybdenum disulfide are used to
lubricate.
India ink, another common laboratory item in the era when
graphs and
diagrams for
publications were drawn by hand and not generated by a
computer, is a colloid of fine
carbon soot in water that also acts as a conductive paint.

Staedtler Mars pen set, circa 1980, that I used to draw many of my early data graphs.
The ink in this set is India ink.
Photograph by the author)
Today,
silver in
lacquer or
epoxy is a common substitute for aquadag and India ink when making connections to a
specimen for
electrical measurement. Such silver-filled inks and
pastes produce layers with a sheet resistance of a few tens of milliohms per square. A common use for these, other than wire connection, is to repair broken conductors in
printed circuit boards.
This leads to the idea that it might be possible to actually "print" your printed circuit board. I wrote in an
earlier article (Silver Ink, January 19, 2012) how
scientists from the
Department of Materials Science and Engineering and the
Frederick Seitz Materials Research Laboratory at the
University of Illinois at Urbana-Champaign have developed a process for this application.[3-4]
The limitation of
inkjet printing a colloid of solid silver particles is that the smallest conductor width is the particle
diameter, which is generally several tens of
micrometers.[3] To overcome this problem, the Illinois research team deposited
liquid precursors for silver based on
silver acetate that yielded 22
wt-% silver.[3] The one disadvantage of the precursor technique is that a
decomposition reaction is required at a
temperature that's too high for most
polymeric substrates.
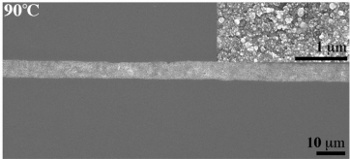
Microstructure of printed silver ink after annealing. The electrical conductivity is the same as for bulk silver.[3]
(Courtesy of S. Brett Walker. Used with permission.)
In
research to produce conductive silver ink that doesn't require a high temperature processing step,
chemists at
Duke University have experimented with a colloid in which the solid silver is in the form of
nanowires.[5-6] The conductivity of printed circuit traces made from this ink far exceeds that for inks made from
nanospheres or microflakes when deposited without a high temperature processing step, thus enabling printed circuitry on
paper and
plastic substrates.[6]
In their
experiments, the Duke chemists examined the
morphology and resistivity of thick films of silver nanowires of two different lengths, silver nanoparticles, and silver microflakes when processed at temperatures from 70-400 °C.[5]
Distilled water was used as the ink
vehicle, and the tests were facilitated with a simple sample preparation method. A
punch was used to create holes in
double-sided tape, which was applied to
glass slides. A precise
volume of the silver nanoparticle ink was injected into these tape wells, the water was
evaporated, and the various temperatures were applied.[6]
With heating to just 70 °C, films of the long silver nanowires had a resistivity of just 1.8 x 10
-5 ohm-cm, so they were 4000 times more conductive than films made from silver nanoparticles.[5] After
sintering at 300 °C, the resistivity of all the silver materials, except microflakes, converged to a value of about 2.5 x 10
-5 ohm-cm, while films of silver microflakes were about 10 times less conductive, having a value of about 4 x 10
-4 ohm-cm.[5] When ten wt-% silver nanowires were added to silver nanoparticle ink, there was a 400-fold improvement in conductivity.[5]
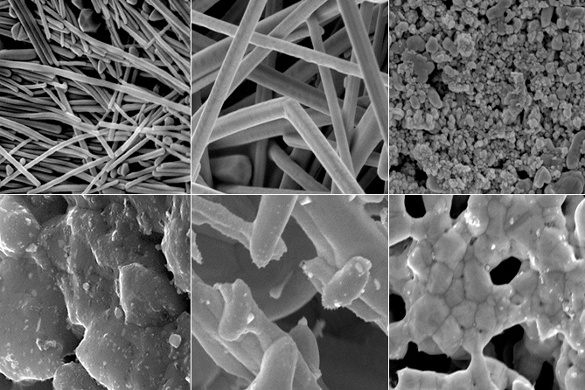
Top row, material processed at 70°C; bottom row, material processed at 300 °C. These images show regions of about two micrometers square in all cases, with significant melting observed at 300°C. (Duke University image by Ian Stewart and Benjamin Wiley.)
These results are significant, and they would allow wide application in
solar cells,
flexible displays,
touchscreens,
batteries and
implantable bio-electronic devices.[6] Says
Benjamin Wiley, an
assistant professor of
chemistry at Duke University and
corresponding author on the
paper describing these results,
"The nanowires had a 4,000 times higher conductivity than the more commonly used silver nanoparticles that you would find in printed antennas for RFID tags... So if you use nanowires, then you don't have to heat the printed circuits up to such high temperature and you can use cheaper plastics or paper... There is really nothing else I can think of besides these silver nanowires that you can just print and it's simply conductive, without any post-processing."[6]
Significantly, the required
quantity of silver needed to make these conductors is small.[6] The Duke research team is experimenting with inkjet printing of conductive circuit traces. Also, silver-coated
copper nanowires, which would be
less expensive than the pure silver nanowires, might display the same conductivities.[6] This research was supported by the
National Science Foundation.[6]
References:
- Ashutosh Jogalekar, "Ernest Rutherford, master of simplicity," Scientific American Blogs, August 30, 2013.
- Data Sheet AGG303: Colloidal Graphite - "Aquadag," Agar Scientific (PDF File).
- S. Brett Walker and Jennifer A. Lewis, "Reactive Silver Inks for Patterning High-Conductivity Features at Mild Temperatures," J. Am. Chem. Soc., (January 5, 2012), DOI: 10.1021/ja209267c.
- Liz Ahlberg, "Particle-free silver ink prints small, high-performance electronics," University of Illinois Press Release, January 12, 2012.
- Ian E. Stewart, Myung Jun Kim, and Benjamin J. Wiley, "Effect of Morphology on the Electrical Resistivity of Silver Nanostructure Films," ACS Appl. Mater. Interfaces, Article ASAP (December 16, 2016), DOI: 10.1021/acsami.6b12289.
- Kara Manke, "Nanowire 'Inks' Enable Paper-Based Printable Electronics," Duke University Press Release, January 3, 2017.
Permanent Link to this article
Linked Keywords: Material; physics; laboratory; Ernest Rutherford (1871-1937); physicist; Nobel Prize in Chemistry; experiment; bicycle handlebar; string; sealing wax; natural leather; gasket; cryogenics; low temperature; proton; director; Cavendish Laboratory; University of Cambridge; Cambridge University; James Chadwick; neutron; Wikimedia Commons; aquadag; colloid; water; graphite; paint; electrical conductor; aqueous solution; dilution ratio; dilute; sheet resistance; ohm; celsius; °C; copper; industry; industrial; electrode; cathode ray tube; electric current; dry lubricant; molybdenum disulfide; lubrication; lubricate; India ink; Cartesian coordinate system; graph; schematic; diagram; scientific literature; publication; computer; carbon; soot; Staedtler Mars; pen; data; silver; lacquer; epoxy; sample; specimen; electrical measurement; paste; printed circuit board; scientist; Department of Materials Science and Engineering; Frederick Seitz Materials Research Laboratory; University of Illinois at Urbana-Champaign; inkjet printing; diameter; micrometer; liquid; precursor; silver acetate; mass fraction; wt-%; chemical decomposition; decomposition reaction; temperature; polymer; polymeric; microstructure; anneal; research; chemist; Duke University; nanowire; nanoparticle; nanosphere; microflake; paper; plastic; experiment; morphology; distilled water; vehicle; hole punch; double-sided tape; microscope slide; glass slide; volume; evaporation; evaporate; sinter; Ian Stewart; solar cell; rollable display; flexible display; touchscreen; battery; microchip implant; implantable bio-electronic device; Benjamin Wiley; assistant professor; chemistry at Duke University; corresponding author; antenna; radio-frequency identification; RFID tag; mass; quantity; cost; less expensive; National Science Foundation.