Flow Batteries
March 12, 2015
Winter is an
energy-intensive time of year at
Tikalon's home in the
Northeastern United States. The
days are shorter, leading to a longer daily interval of
artificial lighting. The newer
LED lamps have reduced
energy cost considerably, but
home heating requires a lot of energy. About 250
therms is needed to heat my house during the course of a cold winter
month. A therm is about 100
cubic feet of
natural gas; and, depending on location, a therm costs slightly more, or less, than a
dollar.
There's also considerable expenditure of
human energy involved in
snow removal. Fortunately, I have a powerful
snow blower. This nine
horsepower blower allows the clearing of my
driveway, and the driveways of two
neighbors, in just an
hour after a typical eight
inch snowfall. The traditional
conversion factor for a horsepower is 746
watts, so a nine horsepower engine is equivalent to more than six
kilowatts! When my home
boiler and snowblower are operating simultaneously, I'm a major contributor to
greenhouse gas emission.
I'm an advocate of
renewable energy, having written quite a few articles on this topic over the years. While
hydroelectric and tidal power plants are renewable energy sources with fairly constant output, the supply of energy from other renewable energy sources is sporadic. You can't get that much solar energy on cloudy days, and there's none at
night.
Wind turbines are capable of supplying power at night, but only when the
wind blows. You need to
store energy so it will be available for later use.
Here's a list of articles on energy storage from this blog:
• Flywheel Energy Storage, July 21, 2011
• Wind-Up Toys, August 5, 2011
• Potential Energy Storage, February 15, 2012
• Flow Batteries, July 18, 2012
• Flow Energy Storage, December 12, 2012
• Compressed Air Energy Storage, July 24, 2013
• The Electrical Grid, September 30, 201
While
batteries are used in some energy storage applications, their low
energy density precludes their use when large quantities of energy must be stored.
Gasoline has an energy density of 46.9
kilojoules per
gram, but a
lithium battery will store just 2.5 kilojoules per gram.
There's a proposal for distributed battery storage of
electricity using
electric vehicle batteries,[1] but an improved battery
technology must be developed before batteries are an
economical component for dedicated
electrical grid energy storage.
Flow batteries might be that technology, but the energy density of presently developed
aqueous flow batteries is lower than that of lithium batteries with
LiFePO4 cathodes (about 225
watt-hour/
liter).[2]
Batteries store energy by
chemical reaction. In the case of a
lead-acid battery, it's through the
reversible transformation of the
lead electrodes to lead sulfate. There are some
electrochemical reactions, however, that involve only
electrolyte solutions, so the electrode materials are not changed. A flow battery operates by removing the charged electrolytes from such batteries, storing them in tanks, then replacing them when battery
discharge is required.
A team of
scientists at the
Pacific Northwest National Laboratory has just
published research on a flow battery based on the reaction of
zinc and
iodine, both of which are relatively benign
materials. The battery
half reactions and overall
reaction are as follow:
(I3)- + 2e- ↔ 3I- (cathode)
Zn ↔ Zn2+ + 2e- (anode)
(I3)- + Zn ↔ 3I- + Zn2+ (overall)
The reaction
potential at the
cathode with respect to a
standard hydrogen electrode is 0.5360
volts, and the potential at the
anode is -0.7626 volts, giving this battery a
cell voltage of 1.2986 volts. A
schematic diagram of this flow battery appears below, along with a photo of the
laboratory demonstration
apparatus for a related
vanadium-redox flow battery.
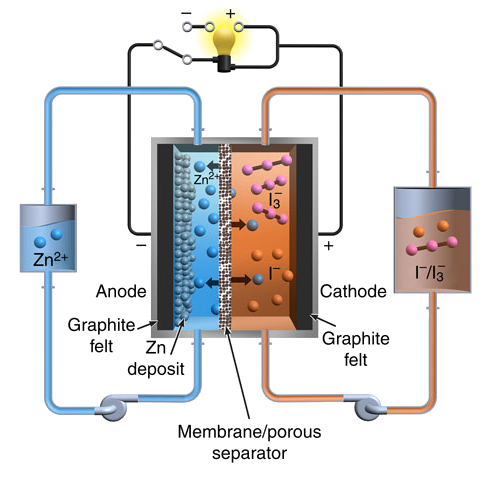
Schematic diagram of the Pacific Northwest National Laboratory zinc-iodine flow battery. (Fig. 1a of ref. 2, licensed under the Creative Commons Attribution 4.0 International License.)
Go with the flow.
Flow battery construction is easy, as this laboratory demonstration of a related vanadium-redox flow battery shows.
Note the safe laboratory practice of placing the flasks in a polyethylene tray to mitigate spills.
(Pacific Northwest National Laboratory image.)
In the discharged state, both tanks contain an electrolyte with a
neutral pH mixture of Zn
2+ cations and I
- anions. In a charged state, one tank contains another negative ion,
polyiodide, I
3-, while zinc ions are passed through the
selective membrane to
electroplate as
metallic zinc on the negative electrode.[2,4]
The electrolyte of the zinc-polyiodide flow battery developed by the Pacific Northwest National Laboratory has an
energy density more than two times that of the next-best flow battery and 70% that of a
lithium iron phosphate battery.[4] Says
Wei Wang, an
author of the study and a
materials scientist at PNNL, "Another, unexpected bonus of this electrolyte's high energy density is it could potentially expand the use of flow batteries into mobile applications such as powering
trains and
cars."[4]
The PNNL team has demonstrated a discharge energy density of 167 watt-hour/liter for a nearly neutral pH 5.0 M ZnI
2 electrolyte.[2] In
theory, a more highly
concentrated electrolyte could discharge up to 322 watt-hour/liter.[4]
Electric cars use about 350 watt-hours for each
mile of
city driving.[4] The following
graph shows the
cycling performance of the zinc-iodine flow battery using
Nafion 115 as the membrane, a 3.5 M ZnI
2 electrolyte, and a
current density of 10
mA/
cm2.[2]
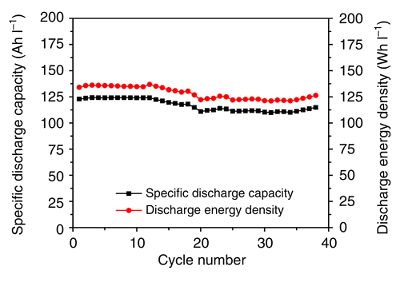
Cycling performance of the zinc-iodine flow battery.
(Fig. 2c of ref. 2, licensed under the Creative Commons Attribution 4.0 International License.)
Even at a lower energy density, the zinc-iodine flow battery has some advantages over lithium cells. First, lithium batteries are not completely
fire-safe, while the water-based (see exception, below) electrolyte in the flow cell won't ignite.[4] Also, the electrolyte isn't
acidic, so it's less
hazardous than the electrolyte in other flow batteries, and in other batteries.[4] In order to extend the operating
temperature range, and minimize zinc
dendrite formation, the PNNL team added
ethanol to the electrolyte. This allowed operation from -20 to 50
°C.[2-4]
Zinc dendrites are a problem, since they can puncture the membrane, so the PNNL team is trying other
alcohols and additives to see whether the dendrites can be eliminated, and the team is scaling-up to a 100-watt-hour system.[4] This research was funded by the
US Department of Energy.[4]
References:
- Mark Chediak, "Musk Battery Works Fill Utilities With Fear and Promise," Bloomberg, December 4, 2014.
- Bin Li, Zimin Nie, M. Vijayakumar, Guosheng Li, Jun Liu, Vincent Sprenkle, and Wei Wang , "Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery," Nature Communications, vol. 6, article no. 6303 (February 24, 2015), doi:10.1038/ncomms7303. This is an open access article, with a PDF file available, here.
- Supplementary information for ref. 2 (850 kb PDF file).
- Frances White, "New flow battery to keep big cities lit, green & safe," Pacific Northwest National Laboratory press release, February 25, 2015.
- How Flow Batteries Work, Pacific Northwest National Laboratory YouTube video, February 25, 2015.
Permanent Link to this article
Linked Keywords: Winter; energy; year; Tikalon; Northeastern United States; day; artificial lighting; LED lamp; cost; central heating; home heating; therm; month; cubic foot; cubic feet; natural gas; dollar; human; snow removal; snow blower; horsepower; driveway; neighbor; hour; inch; snowfall; conversion factor; watt; boiler; greenhouse gas emission; renewable energy; hydroelectricity; hydroelectric; tidal power; renewable energy sources; solar energy; cloud; cloudy; night; wind turbine; electric power; wind; flywheel energy storage; wind-up toys; potential energy storage; flow batteries; flow energy storage; compressed air energy storage; electrical grid; battery; energy density; gasoline; joule; kilojoule; gram; lithium battery; electricity; electric vehicle; technology; economics; economical; electrical grid; aqueous solution; lithium iron phosphate battery; LiFePO4; watt-hour; liter; chemical reaction; lead-acid battery; reversible process; chemical transformation; lead; electrode; lead sulfate; electrochemistry; electrochemical; electrolyte solution; electric charge; charged; discharge; scientist; Pacific Northwest National Laboratory; scientific literature; publish; research; zinc; iodine; material; half reaction; chemical reaction; electrode potential; cathode; standard hydrogen electrode; volt; anode; electrochemical cell; schematic diagram; laboratory; apparatus; vanadium redox battery; Creative Commons Attribution 4.0 International License; polyethylene; neutral pH; cation; anion; polyiodide; ion exchange; selective membrane; electroplating; electroplate; metal; metallic; energy density; Wei Wang; author; materials science; materials scientist; train; automobile; car; theory; concentration; concentrated; electric car; mile; city; driving; Cartesian coordinate system; graph; charge cycle; cycling; Nafion; current density; ampere; mA; square meter; cm2; flammability; fire-safe; acid; acidic; hazard; temperature; dendrite; ethanol; Celsius; °C; alcohol; US Department of Energy.