Thermogalvanic Cell
June 25, 2014
Rules of thumb are nice to have when no other
data are available. The first such rule I learned is, "
a pint's a pound the world round." If you insist on using the
English system of units, as we do in the
United States, this means that a
gallon of
water weighs about eight
pounds, as does a gallon of
milk, and a gallon of nearly every other
liquid.
The actual values are 8.34 lb./gal. for water and 8.6 lb./gal. for
whole milk.
Gasoline is a lightweight, weighing in at just 6.1 lb./gal. Pure
octane has a density of 5.84 lb./gal, while
ethanol has a density of 6.58 lb./gal.
Another rule of thumb that I learned from a
metallurgy professor is that the
density of everything is five. That's because the average density of the
Earth is 5.5, since it's a composite of
crustal rock (density about 2.5), and the density of
iron (about 7.87) comprising its
core.
I wrote about other
scientific rules of thumb in an
earlier article (Rules of Thumb, September 5, 2008). These include the
Dulong and Petit Law, that the
specific heat of a
material is always three times the
gas constant (R) divided by the
molar mass, and the
Wiedemann-Franz Law, that the
ratio of the
thermal conductivity to the
electrical conductivity of a
metal is
proportional to the
temperature.
It's not surprising that there's a
web site,
rulesofthumb.org, devoted to this topic. This site has more than five thousand such rules, just a small fraction of which involve
science and
technology. One of my favorites there is
Rule 1966, "The shorter the life of an
elementary particle, the more it costs to make." Also entertaining is
Rule 1686, "For
mathematics professors, each
published math paper is worth $10,000 in
salary."
Once you have such basic data, it's time for
calculation. As I wrote in
"Estimation" (December 21, 2011),
Nobel physics laureate,
Enrico Fermi was a master at estimating nearly anything, including the number of
piano tuners in
Chicago (in the days when many people still had
mechanical pianos in their
homes). His
back-of-the-envelope calculations would infer the desired quantity by a very long chain of estimated values using simple
models. There was a
book, "Guesstimation: Solving the World's Problems on the Back of a Cocktail Napkin," published on this topic in 2008.[1]
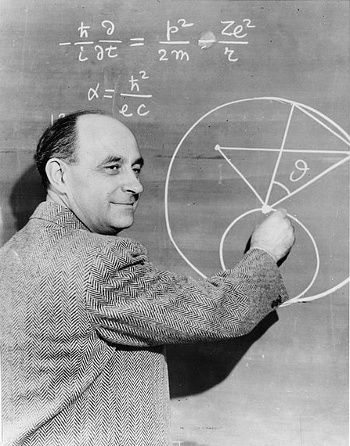
Enrico Fermi at a blackboard.
The expression for the fine structure constant, α, is wrong. Fermi's smile might indicate an intentional joke.
Back-of-the-envelope calculations based on imperfect information are called "Fermi problems" in his honor.
(Smithsonian Institution photograph, via Wikimedia Commons)
All this is preface to an important
chemist's rule of thumb that the
rate of most
reactions doubles every ten
degrees Celsius. This rule is a consequence of the
Arrhenius equation, which expresses the
reaction rate k in terms of an
activation energy Ea and
absolute temperature T; viz.,

Where
R is the
gas constant. This could be called the "chemist's" version of the equation, especially when the gas constant is expressed in
calories as 1.9872 cal/K/mol. The "
physicist's" version of the equation, shown below, uses the
Boltzmann constant kB, instead. The units for the activation energy are different between these equations.

Activation energies vary widely, but a reasonable value is 12.5
kcal. Plugging this into the Arrhenius equation gives a rate change of 1.96 when going from 300
K to 310 K. This large temperature dependence of reaction rate allows us to speed
exothermic reactions by
heating, or slow them by
cooling. Changing the reaction rate also changes the
equilibrium mixture of
products and
reactants.
Scientists from the
Department of Materials Science and Engineering,
Stanford University (Stanford, California), the
Department of Mechanical Engineering,
Massachusetts Institute of Technology (Cambridge, Massachusetts), and the
Stanford Institute for Materials and Energy Sciences,
SLAC National Accelerator Laboratory (Menlo Park, California) have demonstrated that such
thermochemical effects can be used for
thermal energy-harvesting at small differential temperatures. Their thermogalvanic cell is described in a recent issue of
Nature Communications.[2-3]
The operation of such a thermogalvanic cell for energy-harvesting is shown in the figure. An
electrochemical cell is heated so that its
voltage becomes lower. It is then
charged at a higher temperature, using a low voltage. After the cell is cooled, its voltage becomes higher, and it's
discharged at this lower temperature at the high voltage. The
heat energy is harvested from the voltage difference between the high and low temperature states.
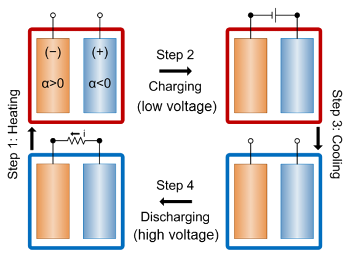
Energy-harvesting cycle of a thermogalvanic cell.
(MIT illustration.)
[3)]
The thermogalvanic cell is proposed for
generating electricity through energy harvesting of the
waste heat available from many processes. This heat is generally available only at a temperature just a hundred degrees
Celsius higher than the
Environment.[3] Energy -harvesting of such heat sources is presently done by low
efficiency thermoelectric devices.[2] MIT's
Gang Chen, an
author of the study, explains that the concept goes back to the 1950s, but
materials at that time could not make a reasonably effective device.[3]
The electrochemical system is based on a
copper hexacyanoferrate cathode and a Cu/Cu2+
anode.[2] The device showed an
energy conversion efficiency of 5.7% in cycling between 10°C and 60°C.[2-3] Says
Yuan Yang, an author of the study and a
postdoctoral associate in MIT's
Mechanical Engineering Department, "One-third of all energy consumption in the
United States ends up as low-grade heat."[3]
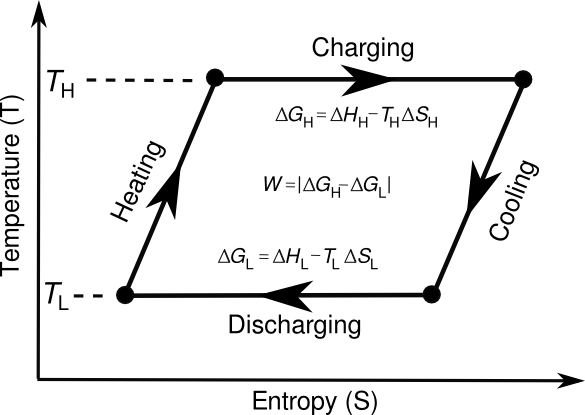
Thermodynamic cycle for a thermogalvanic cell. The cycle is based on the defining equation for the Gibbs Free Energy G; that is, G = H - TS, where H is the enthalpy, and S is the entropy. The theoretical amount of energy that can be harvested is the area defined by the cycle loop. (Drawing by the author using Inkscape.)
This method of thermal energy conversion has several present problems, the first of which is that it doesn't operate against a
temperature gradient as thermoelectrics do; rather, the entire device needs to be heated and cooled in a cycle. Another problem, shared with all
battery systems, is its low
power density. Then there's the speed of charging and discharging.[3]
Funding for this research came from the
US Department of Energy, the
US Air Force, and the
National Research Foundation of Korea.[3]
References:
- Lawrence Weinstein and John A. Adam, "Guesstimation: Solving the World's Problems on the Back of a Cocktail Napkin, Princeton University Press, April 21, 2008, (via Amazon).
- Seok Woo Lee, Yuan Yang, Hyun-Wook Lee, Hadi Ghasemi, Daniel Kraemer, Gang Chen and Yi Cui, "An electrochemical system for efficiently harvesting low-grade heat energy," Nature Communications, vol. 5, article no. 3942 (May 21, 2014), doi:10.1038/ncomms4942.
- David L. Chandler, "A new way to harness waste heat," MIT Press Release, May 21, 2014.
Permanent Link to this article
Linked Keywords: Rule of thumb; data; A pint's a pound the world round; English system of units; United States; gallon; water; pound; milk; liquid; whole milk; gasoline; octane; ethanol; metallurgy; professor; density; Earth; crust; rock; iron; core; science; scientific; Dulong and Petit Law; specific heat; material; gas constant; molar mass; Wiedemann-Franz Law; ratio; thermal conductivity; electrical conductivity; metal; proportional; temperature; web site; rulesofthumb.org; technology; Rule 1966; elementary particle; Rule 1686; mathematics; scientific literature; publish; salary; calculation; Nobel Prize in Physics; Nobel laureate; Enrico Fermi; piano tuning; piano tuner; Chicago; mechanical piano; home; back-of-the-envelope calculation; scientific modelling; book; blackboard; fine structure constant; smile; joke; Fermi problem; Smithsonian Institution; photograph; Wikimedia Commons; chemist; reaction rate; chemical reaction; Celsius; Arrhenius equation; activation energy; absolute temperature; calorie; physicist; Boltzmann constant; kcal; Kelvin; K; exothermic reaction; heating; cooling; equilibrium; product; reactant; Department of Materials Science and Engineering; Stanford University (Stanford, California); Department of Mechanical Engineering; Massachusetts Institute of Technology (Cambridge, Massachusetts); Stanford Institute for Materials and Energy Sciences; SLAC National Accelerator Laboratory (Menlo Park, California); thermochemistry; thermochemical; thermal energy-harvesting; Nature Communications; electrochemical cell; voltage; battery charger; battery; discharge; heat energy; electricity generation; generating electricity; waste heat; environment; energy conversion efficiency; thermoelectric effect; Gang Chen; author; copper; ferrocyanide; hexacyanoferrate; cathode; anode; Yuan Yang; postdoctoral associate; Mechanical Engineering Department; United States; Gibbs Free Energy; enthalpy; entropy; theory; theoretical; energy; energy harvesting; area; Inkscape; temperature gradient; power density; US Department of Energy; US Air Force; National Research Foundation of Korea; Lawrence Weinstein and John A. Adam, "Guesstimation: Solving the World's Problems on the Back of a Cocktail Napkin, Princeton University Press, April 21, 2008.