The Miller Experiment
July 30, 2014
Every
scientist is familiar with the
chemical compound,
urea ((NH
2)
2CO), although not usually in a
laboratory setting. One notable exception from my
undergraduate days was a white-haired
physiology professor who was seen by a
student to be
urinating in a laboratory
sink. Perhaps "
lab alcohol" was involved. I once used urea as an additive to an
electroplating solution.
Urea was first
synthesized from
inorganic reagents by
Friedrich Wöhler in 1828. He reacted
silver cyanate (AgNCO) with
ammonium chloride (NH
4Cl), as follows:
AgNCO + NH4Cl -> (NH2)2CO + AgCl
In Wöhler's time, it was thought that
living things and their
chemical products were fundamentally different from ordinary chemicals. Wöhler's synthesis of urea showed that this concept of
vitalism was wrong. This was the beginning of
organic chemistry, which has subsequently given us useful
materials from
paint to
pharmaceuticals.
Wöhler's synthesis of urea was also an entrée to the idea of
abiogenesis, how life on
Earth could arise from non-living matter. At some point in the
history of the Earth, self-replicating, living matter appeared in
Darwin's "warm little pond,"[1] and it eventually took over its entire
surface. I wrote about abiogenesis and the idea of life on other
planets in an
earlier article (Catalytic Abiogenesis, January 26, 2011).
At first thought, the whole process of abiogenesis seems too improbable to have happened; but, then, where have all the organisms come from?
Theory is one thing, but scientists are skeptical, and that's why they do
experiments. In 1953,
Stanley Miller, who was a
Ph.D. student in
chemistry under
Nobelist,
Harold Urey at the
University of Chicago, performed an
interesting experiment.

Stanley Miller in 1999 with a recreation of his 1953 abiogenesis experiment.
The spark device shown was once common in laboratories for checking glass assemblies for vacuum leaks.
Tens of thousands of volts are generated by a Tesla coil circuit, and any leaks would appear as a spot of light caused by excitation of gas molecules.
(NASA photo by James A. Sugar, via Wikimedia Commons.)
Miller put the supposed components of Earth's early
reducing atmosphere,
ammonia,
methane,
hydrogen and
water, in a closed
reaction vessel, as shown in the figure. He excited this mixture with artificial
lightning produced by a laboratory device capable of producing
high voltage electrical discharges. The reaction produced
amino acids, an essential building block for living organisms.
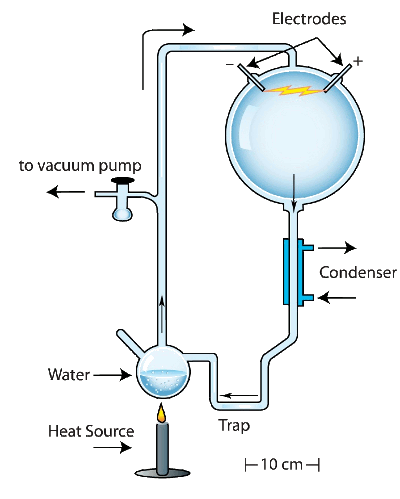
Schematic diagram of Stanley Miller's 1953 abiogenesis experiment.
Illustration by Ned Shaw, Indiana University (modified).)
As
critics of science enjoy pointing out, scientific
theory is changeable. Wait long enough, and your
coffee (or
red wine, or a variety of other
foods), once thought to be bad for your
health, will become a
health food.
Research after Miller's experiment indicates that Earth's early atmosphere was different from Miller's mixture. Instead of ammonia, methane, hydrogen and water, it was more like
carbon dioxide,
carbon monoxide,
nitrogen, and water. Some hydrogen and methane would have come from
volcanic sources, as would lightning.
Miller's experiment got a "reboot" in 2008 when a graduate student discovered sealed
glass vials containing
samples from Miller's original experiments.
NASA, which has invested heavily in astrobiology now that
extrasolar planets are being discovered by the hundreds, funded a reanalysis of the reaction products with today's more
sensitive equipment. One
unplublished reaction simulated conditions near erupting
volcanoes, and the reaction products were found by modern equipment to have produced twenty two
amino acids.[2]
Data mining of Miller's samples and
notebooks has produced some new avenues for investigation. Miller added
cyanamide to his reactions, but he never analyzed the products. Cyanamide is a potential
polymerization agent for amino acids, linking them into the the simple
peptides from
proteins and
enzymes, necessary for life's
biochemistry, are built. A research team from the
Georgia Institute of Technology (Atlanta, Georgia), the NASA
Johnson Space Center (Houston, Texas), the NASA
Goddard Space Flight Center (Greenbelt, Maryland),
The Scripps Research Institute (La Jolla, California), and the
University of California (San Diego, California), has been doing a follow-up study of prebiotic cyanamide reactions.[3-4]

Stanley Miller's cyanamide samples from 1958.
(Scripps Institution of Oceanography, UC San Diego image.)[4)]
Miller's reason for adding cyanamide is unknown, although it's speculated that one of his coffee colleagues may have suggested it.[4] It's well known that
random conversations among scientists from different backgrounds at
universities and large
corporate research laboratories have led to some interesting scientific advances. In Miller's time the chemical consensus was that reactions with cyanamide were only possible in an
acidic environment, which would have been rare on the early Earth.[4]
Jeffrey Bada, who was Stanley Miller's second graduate student and a frequent
collaborator in later years, was given boxes of Miller's samples in 1999. One box was labeled. "electric discharge sample," in Miller's own
handwriting. Says Bada,
"I opened it up and inside were all these other little boxes... I started looking at them, and realized they were from all his original experiments; the ones he did in 1953 that he wrote the famous paper in Science on, plus a whole assortment of others related to that. It's something that should rightfully end up in the Smithsonian."[4]
Fortunately, from a scientific standpoint, these samples were marked with page number references to Miller's
notebooks. These samples were from Miller's electrical discharge experiments with cyanamide while he was at the
College of Physicians and Surgeons of Columbia University in 1958.[4]
These reaction samples from 1958 were analyzed using
liquid chromatography,
ion mobility spectrometry, and
mass spectrometry, and they were found to contain peptides.[3-4] The analysis detected a dozen amino acids, 10
glycine-containing
dipeptides, and 3 glycine-containing
diketopiperazines.[3]
Since
replication of experiments is an important part of science, the research team repeated Miller's cyanamide experiments, albeit with modern versions of the components, and observed similar products, including peptides.[3-4] Other experiments indicated that
Strecker amino-acid synthesis, a series of chemical reactions by which amino acids are formed from
aldehydes or
ketones, plays an important role in such prebiotic synthesis.[3]
The research team acknowledged the assistance of the
archivists of the
Mandeville Special Collections at the
Geisel Library of the University of California, San Diego, in producing Miller's original laboratory notebooks.[3] I wonder whether my former employer would be able to do the same with my many notebooks; or, if that would really matter. This research received support from the
National Science Foundation and the
NASA Astrobiology Program.[3-4]
References:
- As quoted by Darwin's son, Francis, in The life and letters of Charles Darwin, 1887,
"...my father wrote in 1871: "It is often said that all the conditions for the first production of a living organism are now present, which could ever have been present. But if (and oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, &c., present, that a proteine compound was chemically formed ready to undergo stillmore complex changes, at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed."
- David Bricker, Annie Reisewitz, Daniella Scalice, Nancy Neal-Jones and Bill Steigerwald, "Volcanoes May Have Provided Sparks and Chemistry for First Life," NASA Goddard Space Flight Center, October 16, 2008.
- Eric T. Parker, Dr. Manshui Zhou, Dr. Aaron S. Burton, Dr. Daniel P. Glavin, Dr. Jason P. Dworkin, Prof. Dr. Ramanarayanan Krishnamurthy, Prof. Dr. Facundo M. Fern´ndez, and Prof. Dr. Jeffrey L. Bada, "Simultaneous Synthesis of Amino Acids and Simple Peptides on the Primordial Earth," Angew. Chem. Int. Ed., Early View (June 25, 2014). doi: 10.1002/anie.201403683.
- Brett Israel, "Stanley Miller's Forgotten Experiments, Analyzed," Georgia Institute of Technology Press Release, June 25, 2014.
Permanent Link to this article
Linked Keywords: Scientist; chemical compound; urea; laboratory; undergraduate; physiology; professor; student; urination; urinating; sink; ethanol; lab alcohol; electrochemistry; electroplating; aqueous solution; chemical synthesis; inorganic compound; reagent; Friedrich Wöhler; silver cyanate; ammonium chloride; life; Living thing; chemical product; vitalism; organic chemistry; material; paint; pharmaceutical; abiogenesis; Earth; Geologic time scale; history of the Earth; Charles Darwin; lithosphere; planet; theory; experiment; Stanley Miller; Doctor of Philosophy; Ph.D.; chemistry; Nobel Prize in Chemistry; Nobelist; Harold Urey; University of Chicago; Miller-Urey experiment; vacuum; volt; Tesla coil circuit; light; plasma; excitation; gas molecule; NASA; Wikimedia Commons; reducing atmosphere; ammonia; methane; hydrogen; water; chemical reactor; reaction vessel; lightning; high voltage; electrical discharge; amino acid; schematic diagram; Ned Shaw; Indiana University; criticism; critic; theory; coffee; red wine; food; health; health food; research; carbon dioxide; carbon monoxide; nitrogen; volcanic source; glass vial; sample; NASA; extrasolar planet; scientific instrument; sensitive equipment; scientific literature; unplublished; volcano; amino acid; data mining; lab notebook; cyanamide; polymer; polymerization; peptide; protein; enzyme; biochemistry; Georgia Institute of Technology (Atlanta, Georgia); Johnson Space Center (Houston, Texas); Goddard Space Flight Center (Greenbelt, Maryland); The Scripps Research Institute (La Jolla, California); University of California (San Diego, California); randomness; random; university; corporation; acidic; environment; Jeffrey Bada; collaboration; collaborator; handwriting; scientific literature; paper; Science; Smithsonian Institution; College of Physicians and Surgeons of Columbia University; liquid chromatography; ion mobility spectrometry; mass spectrometry; glycine; dipeptide; diketopiperazine; reproducibility; replication of experiments; Strecker amino-acid synthesis; aldehyde; ketone; archivist; Mandeville Special Collections; Geisel Library; National Science Foundation; NASA Astrobiology Program.