Van der Waals Friction
August 12, 2013
 Any article about workplace friction
Any article about workplace friction should start with the
frictional forces between your
automobile tires and the
road surface that enable your getting to work in the first place. We tend to think only about the bad qualities of
friction, such as those causing
engine wear, and forget about the good qualities of friction that enable us to
brake our cars to a stop when we need to. The science of friction is known as tribology, from the Greek words, τριβω (tribo, first person singular form of the verb, "to rub") and λογια (logia, "study of").
When I was a Boy Scout, I learned that rubbing sticks together can produce a fire. I was never successful in my attempts, but I did see it done during a competition. The heat caused by friction is enough to start a fire. As the figure on the left shows, it's also enough to weld metal together in a process called friction welding.
I wrote about friction in two previous articles (Friction and Wear at the Atomic Level, February 6, 2013 and
Friction, February 1, 2012). The
scientific study of friction goes back three hundred years to
Guillaume Amontons, who published the first
theory of
static friction in 1699. Amontons proposed that frictional forces were just those required to raise an object above the height of
surface irregularities.
Unfortunately, Amontons'
equations fail for extremely smooth surfaces, which should have no static friction.
gauge blocks, which are finely dimensioned material blocks with faces
polished to an extreme smoothness will bond together with considerable force when pressed together. Such examples show that some friction arises from
atomic interactions between surfaces. The
coefficient of friction depends on the
material composition of both the sliding object and that of the surface, a friction "couple," which is another indication that atomic forces are at work.
Whenever
scientists think about atomic forces, their first recourse is the simple model proposed by
physicist,
John Lennard-Jones in 1929.[1-2] The equation for the
Lennard-Jones potential, often called the 6-12 law, models repulsive force as the
inverse twelfth
power of atomic separation, and the attractive force as the inverse sixth power of atomic separation. The resultant curve (in blue) is shown in the following figure.
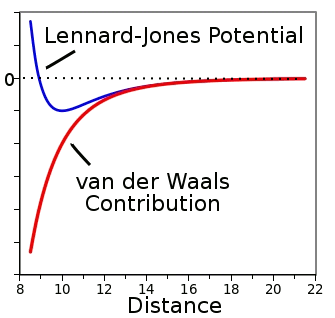
The Lennard-Jones potential as a function of separation for an arbitrary molecule (blue).
The force, which is attractive below the dotted line, tapers to zero at large distances.
(Graph rendered by author using Gnumeric.)
The twelfth power term is a simple consequence of the repulsion that exists when
non-bonding electron orbitals overlap. This happens only at very short range. The always attractive sixth order term, shown in red in the figure, is known as the
van der Waals force. As can be expected when we're talking about interacting
charges, this force is a direct consequence of
molecular dipole moments.
The attractive portion of the Lennard-Jones potential, shown in red in the figure, was derived by
Dutch physicist,
Johannes Diderik van der Waals (1837-1923), who was awarded the 1910
Nobel Prize in Physics "
for his work on the equation of state for gases and liquids." Van der Waal forces are still important a hundred years later, since a search for papers on
arXiv with "van der Waals" in the title gives 299 results.

Johannes Diderik van der Waals (1837-1923)
Van der Waals won the 1910 Nobel Prize in Physics, and his fellow countryman, Heike Kamerlingh Onnes, was awarded the 1913 prize for the liquefaction of helium, which led to his discovery of superconductivity.
(Image modified for artistic effect, via Via Wikimedia Commons.)
Since the van der Waals forces fade as the inverse sixth power of distance, putting a smooth,
amorphous layer on a
crystal should potentially reduce this friction force. Recent
experiments by physicists at
Saarland University and the
Leibniz-Institute for New Materials, both at
Saarbrücken, Germany, were designed to shield the van der Waals forces by such layers to examine this atomic contribution to friction in detail.[3-4] The research team was led by
Karin Jacobs, a physics
professor at Saarland University, and
Roland Bennewitz at the Leibniz Institute for New Materials.[4]
Their model system was
silica (silicon dioxide, SiO
2) on
silicon wafers in which they varied the silica thickness from 1
nm to 150 nm. It was important to maintain the same
surface roughness of the silica layer to eliminate that variable from their analysis. Their friction measurement tool was the 200-nm tip of an
atomic force microscope, which was scanned across the wafer surface.[4]
It was found that the thinnest layer had 30% more friction than the thickest layer. This same effect was observed when the wafers were coated with
silane, a
hydrophobic material.[3-4] What was demonstrated is that the friction of a thin, nanoscale coating depends on the
substrate material; and, also, that the friction of
nanoscale devices can be reduced by a simple method.[4] It was possible to express the experimental data by a
Derjaguin-Müller-Toporov model with appropriate material
parameters.[3]
Said study coauthor, Karin Jacobs,
"The results of our study have significant implications for many practical applications... As the strength of the van der Waals forces depends on the composition of a material to depths of up to 100 nanometres, carefully designing the layer structure at the surface of a material can reduce friction. This gives us another approach to controlling friction in addition to the established use of lubricants."[4]
References:
- J. E. Lennard-Jones, "The Electronic Structure of Some Diatomic Molecules," Trans. Faraday Soc., vol. 25 (1929), pp. 668-686, DOI: 10.1039/TF9292500668.
- G.G. Hall, "The Lennard-Jones paper of 1929 and the foundations of molecular orbital theory,"Advances in Quantum Chemistry, vol. 22, (1991), pp. 1-6; also available, here.
- Matthias Lessel, Peter Loskill, Florian Hausen, Nitya Nand Gosvami, Roland Bennewitz and Karin Jacobs, "Impact of van der Waals Interactions on Single Asperity Friction," Physical Review Letters, vol. 111, no. 3 (July 19, 2013), Document No. 035502 [5 pages].
- Studie: Kontrolle von Reibung durch Massschneidern der van der Waals-Kräfte, Gemeinsame Presse-Info der Universität des Saarlandes und des INM – Leibniz-Institut für Neue Materialien, July 19, 2013; English version, Controlling friction by tuning van der Waals forces, Saarland University Press Release, July 19, 2013.
Permanent Link to this article
Linked Keywords: Social conflict; workplace friction; friction; force; automobile; tire; road surface; engine wear; brake; science; tribology; Greek language; grammatical person; first person singular form; verb; Boy Scouts of America; Boy Scout; fire; welding; weld; metal; friction welding; science; scientific; Guillaume Amontons; theory; static friction; asperity; surface irregularity; equation; gauge block; polishing; polished; atom; atomic; coefficient of friction; material; scientist; physicist; John Lennard-Jones; Lennard-Jones potential; multiplicative inverse; exponentiation; power; Gnumeric; molecular orbital; non-bonding electron orbital; der Waals force; charge; molecular dipole moment; Dutch; Johannes Diderik van der Waals (1837-1923); Nobel Prize in Physics; equation of state for gases and liquids; arXiv; Heike Kamerlingh Onnes; liquefaction of helium; superconductivity; Wikimedia Commons; amorphous solid; crystal; experiment; Saarland University; Leibniz-Institute for New Materials; Saarbrücken, Germany; Karin Jacobs; professor; Roland Bennewitz; silicon dioxide; silica; silicon; wafer; nanometer; nm; atomic force microscope; silane; hydrophobe; hydrophobic; substrate; nanoscopic scale; nanoscale; Derjaguin-Müller-Toporov model; parameter; lubricant.