Bubble, Bubbles, Foam
November 18, 2013
The
bubble pipe was a popular
child's toy of
my generation. but its popularity has waned along with that of
candy cigarettes. A less offensive option is the simple
wire (now
plastic) loop found in the same jar as the
bubble liquid. My first
chemistry experience was the attempt to replicate the bubble liquid
formulation with
household chemicals when the jar's original contents were depleted. Alas,
dish soaps of that era were not as good as those today, so my
experiments were not the successful.

All one shape, but many sizes.
A foam of bubbles on a liquid.
Photograph by Friedrich Böhringer, via Wikimedia Commons)
As I wrote in a
previous article (Bubble Noise Reduction, June 7, 2011), bubbles are useful for more than just toys. A wall of bubbles in
water acts as an effective sound barrier, since there's a large difference in the
product of the
speed of sound v and
density ρ inside and outside the bubbles. This product is the
acoustic impedance Z of the material, and an impedance mismatch causes an
acoustic reflection, just as an
electrical impedance mismatch will cause a
signal reflection in a
transmission line.
The acoustic impedance of
water is more than 3,000 times that of
air, so air bubbles in water will reflect much incident sound, inverting the
phase to cancel the
energy of the incoming
sound wave.[1] Since acoustic
wavelengths are larger than
light wavelengths, large bubbles are preferred, but you can immerse large
balloons for the same effect.[2]
Engineers at
Rice University's Department of Chemical and Biomolecular Engineering have been investigating bubble
foams as a means to enhance
petroleum extraction.[3-5] This
research group, headed by Rice University
associate professor,
Sibani Lisa Biswal, is part of the
Rice Consortium for Processes in Porous Media. A description of this research has just been published in
Soft Matter.[3]
The idea is to use foam as a
displacement volume to extract more petroleum from
underground petroleum reservoirs. The foam would pack highly
permeable channels to force flow through lower permeability channels. The Rice University experiments examined how bubbles transform in a
microfluidic channel. The channel is a
surrogate for the porous media through which oil must flow to be extracted. Their experimental apparatus, as shown in the photograph, forces a
gas-
surfactant mixture through a 20
micrometer channel, which is
imaged 10,000 times per second.[4]

Imaging apparatus for the study of bubble dynamics in a microfluidic channel.
(Still image from a YouTube Video.[5]
The research discovered two new mechanisms of
in situ foam generation in porous media in which an existing bubble is pinched into two bubbles. In one case, a bubble trapped between another bubble and a wall will split as it enters a channel. In the other case, neighboring bubbles break a third bubble into two pieces as it flows through the channel.[4] The width of pinched bubbles was found to obey a
power law in
time.[3]
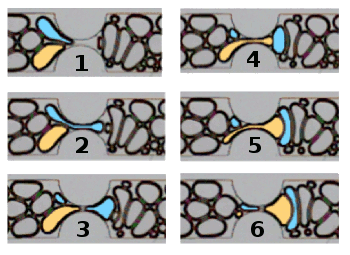
A bubble is split as it enters a microfluidic channel while squeezed between a neighboring bubble and a wall.
(Biswal Lab/Rice University image, modified for clarity.[4]
George Hirasaki, an
emeritus professor of chemical and biomolecular engineering and co-author of the paper, summarized the research by saying,
"We're trying to understand how foam behaves in porous media because it is a way of making gas act like a more viscous fluid... Normally, gas has very low viscosity and it tends to flow through rock and not displace oil and water. Once it finds a path, usually along the top of a reservoir, the rest of the gas tends to follow. If there were some way to make gas act more like a liquid, to make it more viscous, then it would contact much more of the reservoir and would push the fluids out."[4]
This research was funded by various agencies, including the
Petroleum Institute of the United Arab Emirates and the
U.S. Department of Energy.[4]
In my experience,
beer, while technically a
depressant, is a good stimulant for conversations about
physics, and everyone has observed beer foam. Since most beer is today purchased in
cans, not
bottles, few observe an interesting beer foam effect which is the topic of entry no. V102302 in the
American Physical Society Division of Fluid Dynamics Gallery of Fluid Motion 2013.[6-7] As shown in the photograph, tapping one beer bottle vertically with another will result in a beer foam fountain.[6]

Making a beer foam fountain.
Step 1. - Tap an opened bottle at its top with another bottle.
Step 2. - Observe fountain.
(From a video by Javier Rodríguez-Rodríguez, Almudena Casado and Daniel Fuster, via arXiv.[6]
As explained in the video, the vertical
impact of another bottle sends a
compression wave through the liquid, which
resonates between the liquid surface and the bottom of the bottle as an alternating
expansion and compression wave. This leads to
cavitation of the liquid, and small bubbles form to drive the plume.[6]
References:
- B. Würsig, C.R. Greene Jr. and T.A. Jefferson, "Development of an air bubble curtain to reduce underwater noise of percussive piling," Marine Environmental Research, vol. 49, no. 1 (February,2000), pp. 79-93.
- Kevin M. Lee, Kevin T. Hinojosa, Mark S. Wochner. Theodore F. Argo IV and Preston S. Wilson, "Using bubbles to reduce underwater noise," Paper 2aUWb11 of the 161st Meeting of the Acoustical Society of America, May 24, 2011.
- Rachel Liontas, Kun Ma, George J. Hirasaki and Sibani Lisa Biswal, "Neighbor-induced bubble pinch-off: novel mechanisms of in situ foam generation in microfluidic channels," Soft Matter, (Advance Article, September 16, 2013), DOI: 10.1039/C3SM51605A.
- Mike Williams, "Clues to foam formation could help find oil," Rice University Press Release, October 8, 2013.
- Rice University researchers observe two novel ways bubbles form in foam using a high-speed camera, YouTube Video, October 8, 2013.
- Javier Rodríguez-Rodríguez, Almudena Casado and Daniel Fuster, "Why does a beer bottle foam up after a sudden impact on its mouth?" arXiv Preprint Server, October 14, 2013.
- Colin Schultz, "The In-Depth Science of Why a Beer Bottle Erupts When You Whack It," Smithsonian, October 23, 2013.
Permanent Link to this article
Linked Keywords: Bubble pipe; child; toy; baby boomer; generation; candy cigarette; wire; plastic; bubble liquid; chemistry; formulation; household chemical; dishwashing liquid; dish soap; experiment; foam; bubble; liquid; Wikimedia Commons; water; product; speed of sound; density; acoustic impedance; acoustic reflection; electrical impedance; signal; transmission line; air; phase; energy; longitudinal wave; sound wave; wavelength; light; balloon; engineer; Rice University; Department of Chemical and Biomolecular Engineering; foam; petroleum extraction; research; associate professor; Sibani Lisa Biswal; Rice Consortium for Processes in Porous Media; Soft Matter; displacement volume; underground petroleum reservoir; permeability; permeable; microfluidics; microfluidic channel; surrogate model; gas; surfactant; micrometer; digital imaging; YouTube Video; in situ; power law; time; George Hirasaki; emeritus; viscous liquid; viscous fluid; Petroleum Institute of the United Arab Emirates; U.S. Department of Energy; beer; depressant; physics; beverage can; bottle; American Physical Society; Division of Fluid Dynamics; arXiv; impact; compression wave; resonance; rarefaction; expansion; cavitation.