Harder than Diamond
August 31, 2012
Some old
detective movies have a dramatic scene in which someone tests whether a
gemstone is really a
diamond by scratching a
mirror. The misconception is that the only diamond-like material that could scratch
glass is diamond itself.
Quartz, however, looks like diamond, and it has a
Mohs hardness of 7, so it will scratch common glass with a Mohs hardness of about 5.5. Nowadays, you will never see a scene like that in a movie, since
cubic zirconia (Mohs hardness 8-8.5) has become the substitute of choice for inexpensive
jewelry, and it will easily scratch glass.
Cubic zirconia has a
refractive index very close to that of diamond, so the two are
visibly indistinguishable. Diamond has a Mohs hardness of ten, but the real distinguishing property between the two is
thermal conductivity. Diamond is one of the best thermal conductors, and it's used as a
heat sink for some exotic
electronic circuitry.
Cubic zirconia, however, is a thermal insulator. One of my colleagues in the
American Association for Crystal Growth started a company to make cubic zirconia, but he also sold a simple thermal conductivity device to jewelers so they could identify cubic zirconia stones.

Only her jeweler knowns for sure, but any electrical engineer could build a ten dollar thermal conductivity probe that distinguishes cubic zirconia from diamond.
A cubic zirconia gemstone.
(Via Wikimedia Commons))
Diamond has many desirable properties, such as the extreme hardness and thermal conductivity mentioned above. As if as a challenge,
materials scientists strive to produce materials that are harder than diamond. Of course, another motivation is price. They've already produced gem-quality diamonds in the
laboratory[1] after more than a half-century's production of bits and pieces for
abrasives using a
high pressure process; and diamond-like protective coatings by
chemical vapor deposition for things such as
eyeglasses.
Five years ago, I wrote about research on materials that are harder than diamond (
Harder than Diamond, April 24, 2007). There are a few materials that come close.
Cubic boron nitride (β-BN) is nearly as hard as diamond, and it is used also as an industrial abrasive. The likely reason for the hardness of both this material and diamond is the short
covalent bonding between
atoms. Such bonds are particularly
incompressible.
Another material,
beta-carbon nitride (β-C3N4) has the same type of bonding. Its potentially high hardness was suggested in 1985, and it became a target for
synthesis. One commercially viable technique was developed by a research team from the
Shandong University,
People's Republic of China. They synthesized this material using a
mechanochemical reaction technique in which
nanosize graphite powder was first
milled in an atmosphere of
NH3 gas and then
thermally annealed.[2-3]. There are presently no hardness data available for β-C
3N
4.
The compound,
rhenium diboride (ReB2) can be synthesized under
ambient pressures, and a research team at
UCLA prepared ReB
2 and measured its
microindentation hardness in 2007.[4-5] They found an average hardness of 48
GPa, whereas diamond has a hardness of more than 140 GPa. However, the UCLA team found that some of their specimens would scratch diamond, which indicates that ReB
2, an
hexagonal crystal, may be harder than diamond in some of its
crystallographic directions. As an indicator of its incompressibility, the
bulk modulus of ReB
2 was found to be 360 GPa (diamond is 443 GPa).
Of course, the
carbon-carbon bond still has possibilities, as was found in a 2011 study that produced a
glassy carbon structure with an
elastic modulus of 130 GPa.[6-7] This
amorphous carbon allotrope was produced by compressing glassy carbon above 400,000
atmospheres.[6] The material withstood 1.3 million atmospheres pressure in
uniaxial compression under 600,000 atmospheres restraining force in the other directions, something that only diamond can do.[6] The amorphous structure may be an advantage, since such materials have
isotropy. Diamond is stronger in some crystallographic directions that others.[6]
Members of this same research team have recently collaborated with other
scientists to produce a new form of carbon that's harder than diamond.[8-12] The research team was comprised of scientists from the
Carnegie Institution of Washington,
Jilin University (Changchun,China), the
University of Nebraska (Lincoln, NE),
Argonne National Laboratory (Argonne, IL),
Stanford University (Stanford, CA) and the
SLAC National Accelerator Laboratory (Menlo Park, CA).
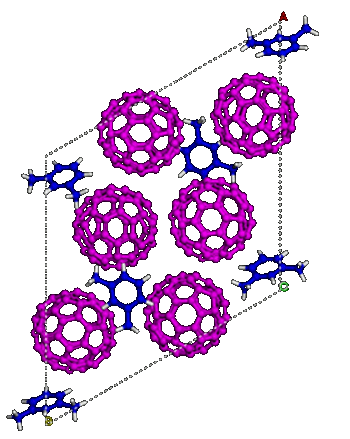
C60 molecules in m-xylene solvent ordered into a lattice.
(Carnegie Institution for Science image. Used with permission.))
This new form of carbon starts with buckyballs (C
60 Fullerene) solvated in
m-xylene (see figure). When pressurized to about 35 GPa, the mixture undergoes an
order-disorder transition.[11] The pressure crushes the buckyballs, but the
solvent molecules remain intact, forming a
lattice that holds everything together; and, once this new
phase is formed, it remains after release of pressure as an incompressible material that can indent diamond.[10]
In their research paper published in
Science,[10] the authors point out that this is the first
hybridization of crystalline and amorphous structures at the atomic level. Says
Lin Wang, lead author of the study,
"We created a new type of carbon material, one that is comparable to diamond in its inability to be compressed. Once created under extreme pressures, this material can exist at normal conditions, meaning it could be used for a wide array of practical applications... The thing that stands out for me from this work is that carbon-60 can crystallize with various solvents, and those solvates would have different periodicities, which enables us to synthesize a series of similar carbon materials with different packing symmetry and carbon cluster size by compressing different types of carbon molecules."[9]
This research was funded by several agencies, including the
US Department of Energy and the
National Science Foundation.[8]
References:
- Ulrich Boser, "Diamonds on Demand," Smithsonian magazine, June, 2008.
- Long-Wei Yin, Mu-Sen Li, Yu-Xian Liu, Jin-Ling Sui and Jing-Min Wang, "Synthesis of beta carbon nitride nanosized crystal through mechanochemical reaction," Journal of Physics: Condensed Matter, vol. 15, no. 2 (January 22, 2003), pp. 309ff.
- L.-W. Yin, Y. Bando, M.-S. Li, Y.-X. Liu and Y.-X. Qi, "Unique Single-Crystalline Beta Carbon Nitride Nanorods," Advanced Materials, vol. 15, no. 21 (November, 2003), pp. 1840-1844.
- Hsiu-Ying Chung, Michelle B. Weinberger, Jonathan B. Levine, Abby Kavner, Jenn-Ming Yang, Sarah H. Tolbert, and Richard B. Kaner, "Synthesis of Ultra-Incompressible Superhard Rhenium Diboride at Ambient Pressure," Science, vol. 316. no. 5823 (April 20, 2007), pp. 436-439.
- Katharine Sanderson, "Scratching diamond just got easier: Ultra-hard material made in the lab without high pressures," Nature Online, April 19, 2007.
- New form of superhard carbon observed, Carnegie Institution of Science Press Release, October 11, 2011.
- Yu Lin,, Li Zhang, Ho-kwang Mao, Paul Chow, Yuming Xiao, Maria Baldini, Jinfu Shu and Wendy L. Mao, "Amorphous Diamond: A High-Pressure Superhard Carbon Allotrope," Phys. Rev. Lett., vol. 107, no. 17 (October 21, 2011), Document No. 175504 (5 pages).
- New form of carbon observed, Carnegie Institution of Science Press Release, August 16, 2012.
- Tona Kunz, "Scientists create new diamond-denting carbon," Argonne National Laboratory Press Release, August 20, 2012.
- Lin Wang, Bingbing Liu, Hui Li, Wenge Yang, Yang Ding, Stanislav V. Sinogeikin, Yue Meng, Zhenxian Liu, Xiao Cheng Zeng and Wendy L. Mao, "Long-Range Ordered Carbon Clusters: A Crystalline Material with Amorphous Building Blocks," Science, vol. 337 no. 6096 (August 17, 2012) pp. 825-828.
- Dongbo Wang and Alejandro Fernandez-Martinez, "Perspective, Materials Science: Order from Disorder," Science, vol. 337 no. 6096 (August 17, 2012) pp. 812-813.
- Dexter Johnson, "New Form of Carbon Dents Diamonds and More," IEEE Spectrum, August 21, 2012
Permanent Link to this article
Linked Keywords: Detective fiction; gemstone; diamond; mirror; glass; quartz; Mohs hardness; cubic zirconia; jewelry; refractive index; light; visible; thermal conductivity; heat sink; integrated circuit; electronic circuitry; American Association for Crystal Growth; Wikimedia Commons; materials science; materials scientist; laboratory; abrasive; synthetic diamond; GE diamond project; high pressure process; chemical vapor deposition; eyeglasses; cubic boron nitride; β-BN; covalent bond; covalent bonding; atom; compressibility; incompressible; beta-carbon nitride; β-C3N4; chemical synthesis; Shandong University; People's Republic of China; mechanochemical reaction technique; nanoscopic scale; nanosize; graphite; milled; ammonia; NH3; thermally annealed; rhenium diboride; ReB2; ambient pressures; University of California, Los Angeles; UCLA; indentation hardness; microindentation; pascal; GPa; hexagonal crystal; crystallographic direction; bulk modulus; carbon-carbon bond; glassy carbon; elastic modulus; amorphous solid; amorphous; carbon allotrope; atmosphere; uniaxial compression; isotropy; scientist; Carnegie Institution of Washington; Jilin University (Changchun,China); University of Nebraska (Lincoln, NE); Argonne National Laboratory (Argonne, IL); Stanford University (Stanford, CA); SLAC National Accelerator Laboratory (Menlo Park, CA); m-xylene; solvent; lattice; fullerene; order-disorder transition; molecule; phase; Science; hybridization; Lin Wang; US Department of Energy; National Science Foundation.