Bonding Teflon®
September 21, 2012
Teflon® is the
trademark for
polytetrafluoroethylene (PTFE), known for its utility as a
high temperature non-stick coating for things such as
cookware. PTFE is non-stick because it has a low
surface energy. Everyone's first question about PTFE coated cookware is, how do they get it to stick to the
metal pan?
The PTFE is mechanically held to the metal by first creating a roughened metal surface, either by
sandblasting, or by
flame spraying a rough metal coating. The PTFE layer interlocks with the pores in the rough surface, so it's mechanically fixed. Not very elegant in a
chemist's viewpoint, but it works just the same.
Plumber's helper.
A common use of PTFE is as a thread sealant and lubricant for piping.
(Photo by author)
There's a way to bond PTFE articles, such as sheets, to other materials by
chemical modification of the PTFE surface to be bonded. The idea is to strip away the
fluorine atoms, leaving a
carbon layer that's bondable with an
adhesive. This is difficult, since the
carbon-fluorine bond is extremely strong. Fluorine is highly
electronegative, and the electronegativity difference between carbon and fluorine is 1.5. The highly
ionic character of this
bond leads to a high
bond-dissociation energy.
Sodium, with an electronegativity of slightly less than one, is reactive enough to pull fluorine away from PTFE; that is, it will etch the material to make a bondable surface. There are various sodium chemistries to accomplish this, some of which are more friendly than others,[1] but other methods for PTFE bonding are always welcome.
A team of
scientists from the
Institute for Materials Science, the
Otto-Diels-Institute for Organic Chemistry, and the
Department of Functional Morphology and Biomechanics at the
University of Kiel (Kiel, Germany) have developed a novel method by which they bonded Teflon with another slippery material, cross-linked
poly(dimethylsiloxane), a
silicone adhesive. In a
microscale-nanoscale variation of the mechanical bonding process, they used small,
tetrapod crystals of
zinc oxide as a bonding agent (see figure). [2-4]
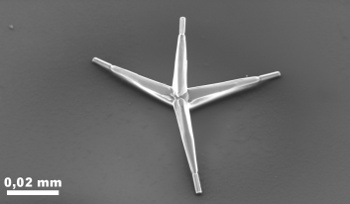
The zinc oxide crystals are tetrapods, as this scanning electron micrograph shows.
(Image by Xin Jin, Copyright Christian-Albrechts-Universität zu Kiel).[3)]
Their extremely simple process involves sprinkling these crystals between sheets of the
polymers, then heating gently at 100 °
C for 40 minutes. This bond is reasonably strong, since a peeling
force of 200
N/
m is required to peel the layers apart.[2,4] This is about the force needed to unstick
household cellophane tape.[2-3]
Not surprisingly, the tetrapodal shape of the crystals is an ideal shape for such interlocking.[4] Pulling on one arm of the tetrapod causes the other three arms to dig into the material more deeply.[3]
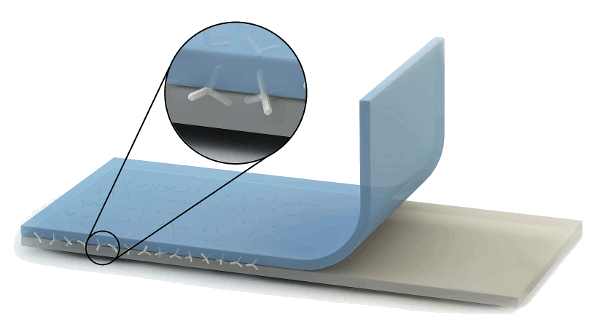
Conceptional drawing of a cross-linked poly(dimethylsiloxane) layer (blue) bonded to PTFE. (Image by Jan Strüben, Copyright Christian-Albrechts-Universität zu Kiel).[3)]
"...as far as we could verify, no one has ever made silicone and Teflon stick to each other at all”, says study co-author Lars Heepe, a graduate student at Kiel University who measured the adhesion.[3]
The PTFE-poly(dimethylsiloxane) couple was an extreme test of the process. Says
Professor Rainer Adelung, a
materials scientist who worked with the research team to develop this process, "If the nano staples make even extreme polymers like Teflon and silicone stick to each other, they can join all kinds of other plastic materials."[3]
Since this is a mechanical adhesion, and zinc oxide is a benign material, this would be a
biocompatible process suitable for
medical implants.[3] It may be possible also to switch the adhesion on and off using
light.[3]
References:
- The Etching of Fluoropolymers In Preparation for Bonding, Acton Technologies, Inc.
- Materials-Sticking the unstickable, Nature, vol. 489, no. 7414 (September 6, 2012), p. 9.
- Joining the Un-Joinable - New polymer linking technology based on nano crystals developed in Kiel, University of Kiel Press Release No. 237/2012, August 24, 2012.
- Xin Jin, Jan Strueben, Lars Heepe, Alexander Kovalev, Yogendra K. Mishra, Rainer Adelung, Stanislav N. Gorb and Anne Staubitz, "Joining the Un-Joinable: Adhesion Between Low Surface Energy Polymers Using Tetrapodal ZnO Linkers," Advanced Materials, vol. 24, no. 35 (2012), DOI: 10.1002/adma.201201780.
Permanent Link to this article
Linked Keywords: Teflon®; trademark; polytetrafluoroethylene; high temperature; adhesion; non-stick; cookware; surface energy; metal; abrasive blasting; sandblasting; thermal spraying; flame spraying; chemist; plumber; piping; chemical reaction; chemical modification; fluorine; atom; carbon; adhesive; carbon-fluorine bond; electronegativity; electronegative; ionic character; chemical bond; bond-dissociation energy; sodium; scientist; Institute for Materials Science; Otto-Diels-Institute for Organic Chemistry; Department of Functional Morphology and Biomechanics; University of Kiel (Kiel, Germany); poly(dimethylsiloxane); silicone adhesive; microscale; nanoscale; tetrapod; crystal; zinc oxide; scanning electron microscope; electron micrograph; Xin Jin; Christian-Albrechts-Universität zu Kiel; polymer; Celsius; C; force; Newton; N; meter; m; household cellophane tape; Jan Strüben; Professor Rainer Adelung; materials scientist; biocompatibility; biocompatible; medical implant; light.