Icing-Resistant Surfaces
August 8, 2012
The
solid phase of
water (i.e.,
ice/
snow) is a major problem for people living in the higher
latitudes.
Northern New Jersey, where Tikalon is located, had a major problem with an early snowstorm at the end of last year.
The snowstorm, which I described in a
previous article (Oh No! - October Snow!, October 31, 2011) happened very early in the season, so the snow was wet and heavy, and
trees still had most of their
leaves. As a result, there were there were many fallen trees and tree limbs taking down
power lines. We were without
power for more than two days.[1]
Ice is a major problem for
aviation. I worked on some aspects of
aircraft icing in my career in
industrial research. I conducted a study of the possibility of using
weather radar to detect potential icing conditions in the
atmosphere. This involved detection of conditions for which
supercooled water would be present. I later developed an icing
sensor.[2] Since ice formation on cold surfaces is difficult to prevent, aviation icing technologies tend towards detection and removal, not prevention.

A 1983 icing test of a model of a commuter aircraft at NASA's Glenn Research Center.
A cold water spray freezes onto the aircraft model, simulating icing in flight.
(NASA Image))
Removal and subsequent delay of the additional formation of ice for aircraft on the ground is done by spraying the aircraft with
antifreeze solutions. In the past, a typical antifreeze agent,
eyhylene glycol, was used. This is the same antifreeze used in the
cooling systems of
motor vehicles. There's been an effort to replace this
toxic ethylene glycol with
propylene glycol, a chemical safe enough to be used as a
food additive. Propylene glycol works just as well as an antifreeze as ethylene glycol (see figure). However, propylene glycol is more expensive.
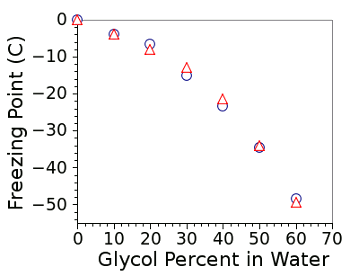
Freezing points of glycol-water solutions.
The circles are data for ethylene glycol, and the triangles are data for propylene glycol.
(Graphed by author using Gnumeric)
Of course, the best way to prevent ice formation on a surface is to prevent water from attaching there. That's the purpose of
hydrophobic surface treatment. As its name implies, a hydrophobe, from the combination of Greek words meaning "fear of water," resists attachment of water droplets. Better than a hydrophobe is a
superhydrophobe, for which the
contact angle of the droplet is very large (>150°).
A large contact angle means less of the water droplet is in contact with a surface, so there is less likelihood that the drop will remain sessile. In the case of an aircraft in flight, for which there is a constant flow of air driving water from the
airframe, droplets with high contact angle will be swept away.
Superhydrophobic materials are produced by using
nanotechnology to
mimic the particular physical structure on the surface of the water-repellent
leaves of the
lotus flower (see figure). This creates a high contact angle, not by the
chemical properties of the liquid and surface, but simply by forcing it mechanically. The
lotus effect works for the lotus plant, and it works for
nanoscale modified surfaces that mimic the surface structure of its leaves.
(Portion of a computer graphic image by William Thielicke, via Wikimedia Commons)
The lotus effect works well for the occasional liquid drop, but not so well under very
humid and icing conditions. In fact, the
roughness of the nanoscale modified surface can lead to
worse icing. A research team of scientists from
Harvard University and the
University of Puerto Rico has modified such superhydrophobic surfaces with the infusion of a
water-immiscible liquid to solve this problem.[3-4] The team is led by
Joanna Aizenberg, a professor of
materials science at Harvard's
School of Engineering and Applied Sciences.[3]
The method, which they call SLIPS, for "Slippery Liquid Infused Porous Surfaces," presents a
molecularly flat liquid interface to oncoming water. The liquid is held in place by the nanostructured surface, which is chemically-treated to have a high affinity for the liquid to hold it in place.[4] Water droplets, frost and ice can't adhere to the liquid, so they slide off.[3]
The liquid infiltrant is non-toxic, and it acts as an
anti-corrosive agent.[3] The research team has shown that SLIP-modified
aluminum has an
order of magnitude lower ice adhesion than other anti-icing materials.[4] If these results hold in an actual working environment, the SLIPS process will help to guard against icing in aircraft,
wires, and
wind turbines.[4]
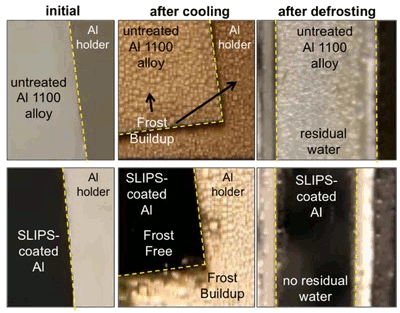
Performance of SLIPS-modified aluminum.
SLIPS delays ice accumulation, and it facilitates ice removal.
(Harvard School of Engineering and Applied Sciences image))
Said team leader, Aizenberg,
"Unlike lotus leaf-inspired icephobic surfaces, which fail under high humidity conditions, SLIPS-based icephobic materials, as our results suggest, can completely prevent ice formation at temperatures slightly below 0°C while dramatically reducing ice accumulation and adhesion under deep freezing, frost-forming conditions...This new approach to icephobic materials is a truly disruptive idea that offers a way to make a transformative impact on energy and safety costs associated with ice, and we are actively working with the refrigeration and aviation industries to bring it to market."[3]
Icing of aircraft while in flight generally occurs at much lower
temperatures than 0°C, so further work needs to be done to have the SLIPS process work for airframes. There's also the problem of
erosion of the nanostructured surface during flight by things such as airborne fine particles. This research was supported by the
National Science Foundation, which also supports
Harvard's Center for Nanoscale Systems.[3]
References:
- 2 Million Without Power in Northeastern US After Rare Snowstorm, Voice of America, October 31, 2011.
- Devlin M. Gualtieri, "Aircraft Icing Sensor, U.S. Pat. No. 7,683,791, March 23, 2010.
- Michael Patrick Rutter and Twig Mowatt, "A new spin on antifreeze - Researchers create ultra slippery anti-ice and anti-frost surfaces," Harvard School of Engineering and Applied Sciences Press Release, June 11, 2012.
- Philseok Kim, Tak-Sing Wong, Jack Alvarenga, Michael J. Kreder, Wilmer E. Adorno-Martinez and Joanna Aizenberg, "Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance," ACS Nano, Article ASAP, June 10, 2012, DOI: 10.1021/nn302310q.
Permanent Link to this article
Linked Keywords: Solid phase; water; ice; snow; latitude; Northern New Jersey; tree; leaf; leaves; power line; electrical power; aviation; aircraft icing; research and development; industrial research; weather radar; atmosphere; supercooled water; sensor; NASA's Glenn Research Center; antifreeze; eyhylene glycol; cooling system; motor vehicle; toxicity; toxic; propylene glycol; food additive; Gnumeric; hydrophobe; hydrophobic; superhydrophobe; contact angle; airframe; nanotechnology; biomimicry; Nelumbo; lotus flower; chemical; lotus effect; nanoscale; Wikimedia Commons; humidity; humid; surface roughness; Harvard University; University of Puerto Rico; miscibility; water-immiscible; Joanna Aizenberg; materials science; School of Engineering and Applied Sciences; molecule; molecular; anti-corrosive; aluminum; order of magnitude; electric power transmission; wire; wind turbines; Harvard School of Engineering and Applied Sciences; temperature; erosion; National Science Foundation; Harvard Center for Nanoscale Systems.