Wind-Up Toys
August 5, 2011
In the days before small, powerful,
permanent magnet motors powered by small
batteries with
high energy capacity, children's toys used mechanical
energy storage. The toy motors could be as simple as the
rubber bands in
throw-away toys found in cereal boxes, or coiled
steel springs in store-bought toys.
A coiled steel spring for use as a wind-up energy storage unit was patented in 1910 as an
automobile engine starter,[1] and springs like these were even used to spin-up
gyroscopes in short term applications, such as
missiles.[2] They were still used in gyroscopes at least to about 1995, since I knew a young
mechanical engineer who did some gyroscope spring calculations at that time.
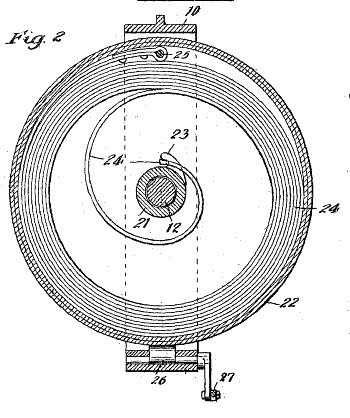
Figure two from US Patent No. 950,848, "Gasolene-Engine Starter," by Edwin A. Gardner, March 1, 1910)
In the automobile engine application, the spring and the engine drive could share the same shaft.
Clocks and
phonographs needed a different arrangement that was patented in 1936, as shown below.
![]()
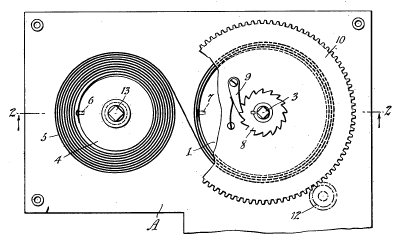
Figure one from US Patent No. 2,063,799, "Spring Motor," by Henry Axel F. Fornelius and Henry A.G. Fornelius, December 8, 1936)
The idea of using mechanical deformation as a means of energy storage can be taken to extremes. One US patent application looks forward to the time at which we can store mechanical energy at a molecular level, in
carbon nanotubes; viz,[4]
"An energy storage device comprising: at least one nanotube; and an energy storage and recovery mechanism for applying the appropriate levels of strain on said at least one nanotube to produce stored energy and relaxing said at least one nanotube to recover said energy for use external to said energy storage device."
This patent application states that steel springs have a
volumetric energy density of about 600
Joule per
liter, whereas the value for a nanotube spring is a phenomenal 11.25 mega-Joule per liter.
This estimate follows from the early work of S. A. Chesnokov, et al.,[5] who reversibly compressed purified, unoriented
single-wall carbon nanotubes at 29
kbar pressure to a
density that approached that of
graphite. They determined that the
reversible work done in flattening the tube cross section from circular to elliptical was 0.18
electronvolt (eV) per
carbon atom. Cranking through the numbers for this random assemblage gives about 3 mega-Joule/liter.
Another means of mechanical energy storage is the
flywheel. I wrote about flywheel energy storage in an article about
"green" braking systems (Green Braking, October 20, 2010). I mentioned flywheels also in
another article (Portable Power Challenge, September 17, 2007) about a 2007
US Defense Department challenge contest for a wearable 20-
watt average power source, operable over four days, and weighing less than four
kilograms (8.8
pounds).
One thing that the DOD challenge shows is how
chemical energy storage bests all other kinds. Twenty watts over four days is 6,912,000 watt-sec, or 6,912 kilojoule (kJ). Listed below are the ultimate energy storage capabilities of various physical or chemical systems (
ignoring external components),[6] and the
mass required to store 6,912 kJ.
Obviously, when a country exports gasoline or ethanol, it's actually exporting energy. Less obvious is the idea that export of
aluminum is also an export of a huge amount of energy. Synthesis of aluminum by the
Hall–Héroult process is energy intensive. The feedstock, which is
aluminum oxide, is dissolved in molten
sodium hexafluoroaluminate (Na
3AlF
6) at about 1000°C. The resulting liquid is
electrolyzed to produce molten aluminum.
Aluminum is
recycled because of the energy needed to refine it, not because of its scarcity. The energy required to refine aluminum is about 14 kilowatt-hour per kilogram.[7] Thus, each
metric ton of aluminum is the equivalent of 14 megawatt-hours of electricity! Think twice before discarding that aluminum beverage can!
References:
- Edwin A. Gardner, "Gasolene-Engine Starter," US Patent No. 950,848, March 1, 1910.
- Joseph J. Moravek and Rocco N. Figalora, "Gyroscope Apparatus," US Patent No. 3,270,568, September 6, 1966.
- Henry Axel F. Fornelius and Henry A.G. Fornelius, "Spring Motor," US Patent No. 2,063,799, December 8, 1936
- Timothy F. Havel and Carol Livermore-Clifford, "Devices For Storing Energy In The Mechanical Deformation Of Nanotube Molecules And Recovering The Energy From Mechanically Deformed Nanotube Molecules," US Patent Application No. 20080305386, December 11, 2008.
- S. A. Chesnokov, V. A. Nalimova, A. G. Rinzler, R. E. Smalley and J. E. Fischer, "Mechanical Energy Storage in Carbon Nanotube Springs," Physical Review Letters, vol. 82, no. 2 (January 11, 1999), pp. 343-346.
- Energy Density page on Wikipedia.
- Pieter van Pelt, "Cheap electricity from Iceland - Giant Aluminum Batteries to transport electricity," ZPEnergy, April 24, 2004
Permanent Link to this article
Linked Keywords: Permanent magnet motor; battery; energy capacity; energy storage; rubber band; cereal box prize; steel spring; automobile; engine starter; gyroscope; missile; mechanical engineer; US Patent No. 950,848; clock; phonograph; US Patent No. 2,063,799; carbon nanotube; volumetric energy density; Joule; liter; single-wall carbon nanotube; kbar; pressure; density; graphite; reversible work; electronvolt (eV); carbon; flywheel; "green" braking system; US Defense Department; watt; kilogram; pound; chemical; mass; flywheel; torsion spring; lead acid battery; nickel-metal hydride battery; lithium battery; supercapacitor; thermite; gasoline; ethanol; hydrogen; lithium; magnesium; sodium; aluminum; Hall–Héroult process; aluminum oxide; sodium hexafluoroaluminate; electrolysis; recycling; metric ton; US Patent No. 3,270,568; US Patent Application No. 20080305386.