Phase Change Conductance
June 21, 2011
In a
previous article (Nano Memory, September 30, 2010), I wrote about memory devices being developed from the reversible change in
electrical conductance that some materials have when they change phase between
amorphous and
crystalline states. This effect was discovered by
Stanford R. Ovshinsky, and it's often called The Ovonic effect.[1] This effect has become important as a memory technology known as
phase-change memory.
Materials that exhibit this effect are quite exotic, and also quite
toxic. An important class of such materials is the
chalcogenide glasses, specifically
selenides and
tellurides, such as Ge
2Sb
2Te
5 (called GST). The amorphous state of these compounds has low conductivity, and the crystalline state has high conductivity.
Application of proper voltages switches between these phases in a few tens of
nanoseconds. This is remarkable, since these transitions involve an actual heating of the material. Such rapid transitions are possible, since the amount of material is very small, and it's in intimate contact with a
heat-sinking substrate.
There are other ways to change the conductance of a material, although not through a change in the intrinsic electrical properties of the material itself, but the conductance path through crystalline grains of the material, a process called
percolation.[3-4] I wrote in two previous articles[5-6] how conductance in such materials can be changed by mechanical
stress, an effect called
piezoresistance.
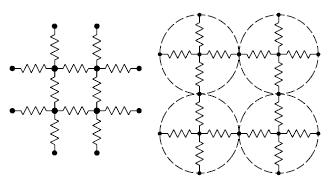
One way to model electrical percolation in two-dimensions.
Particles are modeled as resistive links to nearest neighbors.
Fig. 1 of ref. 4, via arXiv
An historical application of piezoresistance is the
carbon button microphone, which uses a discovery of
Thomas Edison that
sound pressure can change the resistance of
carbon particles placed between conducting plates. Sound pressure causes contact between the loose particles and a resultant
current could flow.
Some
composite materials consisting of a
polymer matrix loaded with conducting particles change resistance also with applied
stress.
Aerogels composed of
multi-walled carbon nanotubes, a material that could be called "frozen smoke," allow reversible compression to just 5% of their volume. The conductivity of these aerogels is highly sensitive to applied
pressure.[6]
The phase change most familiar to people is the
melting of
ice and
freezing of
water. Pure water has very little conductivity, so such a phase change isn't reflected in a useful change in conductance. There is, however, a large change in
volume, and a huge change in
dielectric constant. The dielectric constant of water is about 80, and that of ice only 3.2 at radio frequencies.[7]
The freezing transition has been put to good use by
scientists at
MIT who have used the
phase separation of a two-phase mixture to effect a large increase in both electrical and
thermal conductivity upon freezing.[8-9] They were able to effect a change in electrical conductivity by a factor of more than a hundred; and thermal conductivity by a factor of more than three.
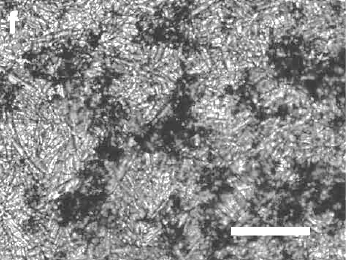
Fig. 1(f) from ref. 7. An image of a network of graphite particles formed by freezing a graphite suspension in hexane. Graphite clusters are black, and the needle-like structure is the frozen hexane. The scale bar is 200 μm.
(Image licensed by the authors of ref. 9 under a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.)
Their material was a
suspension of
graphite flakes, 0.2% by volume, in
hexane (C
6H
14). The researchers found that this suspension was maintained for at least three months. As shown in the figure above, the frozen hexane pushes the graphite flakes together, forming a percolation network.
When hexane freezes, it forms needle-like structures that not only segregate the graphite flakes, but also push against them to increase their contact areas and decrease resistance. Hexane is not the most desirable
solvent, since its
melting point is quite low (-95 °C), but the MIT team has demonstrated the concept with solvents that have a melting point near
room temperature.[8]
One possible application of this material system would be automatically resetting
fuses. This research was led by
Gang Chen, Director of the MIT
Pappalardo Micro and Nano Engineering Laboratories. A U.S. patent application has been filed on this invention, "Thermal and/or electrical conductivity control in suspensions," Application No. 12/720,382.[9]
References:
- Stanford R. Ovshinsky, "Reversible Electrical Switching Phenomena in Disordered Structures," Physical Review Letters, vol. 21, no. 20 (1968), pp. 1450-1453.
- David Lammers, "Resistive RAM Gains Ground," IEEE Spectrum (September 2010).
- A. S. Ioselevich and D. S. Lyubshin, "Percolation with excluded small clusters and Coulomb blockade in a granular system," arXiv Preprint Server, October 15, 2009.
- Yakov M. Strelniker, Shlomo Havlin, Richard Berkovits and Aviad Frydman, "Resistance distribution in the hopping percolation model," arXiv Preprint Server, June 7, 2005.
- This Blog, Piezomolecular Effect, February 22, 2011.
- This Blog, Carbon Nano-Aerogel, March 14, 2011.
- D.M. Gualtieri, "Aircraft icing sensor," U.S. Pat. No. 7,683,791, March 23, 2010.
- David L. Chandler, "New method found for controlling conductivity - Reversible control of electrical and thermal properties could find uses in storage systems," MIT Press Release, April 29, 2011.
- Ruiting Zheng, Jinwei Gao, Jianjian Wang and Gang Chen, "Reversible temperature regulation of electrical and thermal conductivity using liquid–solid phase transitions," Nature Communications, vol. 2, no. 4 (April 19, 2011), Article no. 289, pp. 1-6.
Permanent Link to this article
Linked Keywords: Electrical conductance; amorphous; crystalline; Stanford R. Ovshinsky; phase-change memory; toxic; chalcogenide glasses; selenide; telluride; nanosecond; heat-sinking; percolation; stress; piezoresistance; carbon button microphone; Thomas Edison; sound pressure; carbon; electric current; composite material; polymer; stress; aerogel; multi-walled carbon nanotube; pressure; melting; ice; freezing; water; volume; relative permittivity; dielectric constant; scientist; Massachusetts Institute of Technology; MIT; phase separation; thermal conductivity; suspension; graphite; hexane; solvent; melting point; room temperature; fuse; Gang Chen; Pappalardo Micro and Nano Engineering Laboratories; arXiv; U.S. Pat. No. 7,605,585.