Solar Ceria
July 7, 2011
One fundamental principle of
thermodynamics is that you need a
temperature difference to do
work. This was discovered in 1824 by
Sadi Carnot in his investigation of
heat engines, and this principle is codified in the
second law of thermodynamics. Carnot defined a maximum
efficiency,
η, as
η = 1 - (TC/TH)
where T
C is the temperature of a cold reservoir, and T
H is the temperature of a hot reservoir. When there is no temperature differential, T
H is equal to T
C, and the efficiency is zero.
This idea that a temperature differential provides work is nicely illustrated in the
drinking bird toy that I showed in a
previous article (Second Law of Thermodynamics, February 7, 2011). That article also discussed the second law and the impossibility of
perpetual motion machines. The differential temperature in the drinking bird is provided by
evaporative cooling that provides a slightly cooler temperature below ambient. There's a temperature differential, so work (the dipping action) can be done. Hot engines work best, which is somewhat of a battle cry for
turbine engine designers.
When we want to harvest
solar energy,
photovoltaics are a good choice for small to medium-sized installations. The largest economical photovoltaic array would be one that powers a large building.
Googleplex is a good example, since it has a solar installation that generates 1.6
megawatts, which is about 30% of the power demand for this corporate center.[1]
Solar thermal is the next step in power generation. In a
recent article (Solar Nevada, June 6, 2011), I discussed the Crescent Dunes Solar Energy Project being constructed near
Las Vegas that is rated at 110-megawatts. This installation uses mirrors to focus light onto a reactor that heats molten salt. The heat from the
molten salt is used to create steam to drive a turbine generator. There's another solar thermal method that's being developed by materials scientists at the
California Institute of Technology (Pasadena, CA) that makes use of the thermodynamics of the equilibrium between
cerium oxide and its formative
elements.[2-3]
Cerium oxide, a stable oxide that's also called ceria, is formed from the elements in a highly
exothermic reaction:
Ce + O2 -> CeO2
Stable, of course, is a relative term, since, at a given temperature, there is always a small fraction of unreacted cerium available and an associated
partial pressure of
oxygen. At room temperature, the equilibrium pressure of oxygen over ceria is laughably small. It's just 2.3 x 10
-180 atmospheres, so the idea that ceria is a very stable oxide is well founded.
When the temperature is increased, ceria starts to decompose, although just very slightly. The graph below shows the partial pressure of oxygen above ceria as a function of temperature, as calculated from its
free energy of formation.[4]
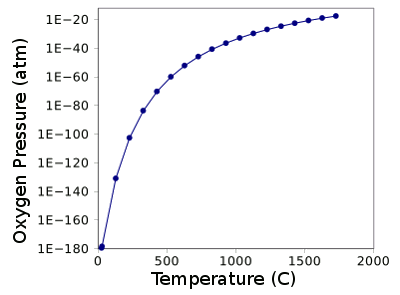
Partial pressure of oxygen above ceria as a function of temperature, as calculated from the Gibbs Free Energy of formation.
Free energy data from Ref. 4.
Graph rendered by Gnumeric)
The idea that ceria will become oxygen deficient at high temperatures and will equilibrate by absorption of oxygen at low temperatures can be utilized to
reduce water and
carbon dioxide to form such useful products as
hydrogen and
carbon monoxide.
Hydrogen and carbon monoxide are precursors for the creation of
syngas, which, in turn, is a precursor for liquid
hydrocarbons.
Catalysts will also convert hydrogen and carbon monoxide to
methane. Using solar energy as the heat source makes this a solar energy process. A schematic of this process is shown in the figure, below.
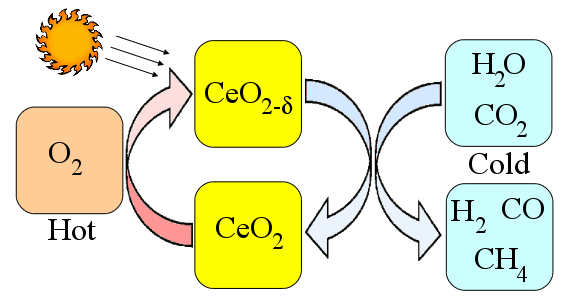
Solar ceria thermal cycle.
The hot-side temperature is about 1650°C. Although the CalTech team obtained only a 0.7 to 0.8% efficiency in their process, they've estimated a 16-19% efficiency when things are optimized.[2] Even this higher percentage is a middling range for solar energy conversion, but the process might be an easy method of
carbon sequestration.
If the carbon dioxide produced at a
fossil fuel plant is processed to produce more
carbon-containing fuel that's used at the plant, it would be a closed-cycle solar system.[3] The hydrogen production rate of 8.5–11.8 ml/gram-ceria is about the same as that of other solid-state thermochemical cycles, but one problem is the need for an extremely
inert atmosphere at the oxygen release stage.[2]
References:
- Court Rye, "Googleplex Solar," Solar Power Authority, March 30, 2009.
- William C. Chueh and Sossina M. Haile, "A thermochemical study of ceria: exploiting an old material for new modes of energy conversion and CO2 mitigation," Phil. Trans. R. Soc. A, vol. 368 no. 1923 (July 28, 2010), pp. 3269-3294
- William C. Chueh, Christoph Falter, Mandy Abbott, Danien Scipio, Philipp Furler, Sossina M. Haile and Aldo Steinfeld, "High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria," Science, vol. 330 no. 6012 (December 24, 2010), pp. 1797-1801.
- L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
Permanent Link to this article
Linked Keywords: Thermodynamics; temperature; work; Sadi Carnot; heat engine; second law of thermodynamics; efficiency; drinking bird toy; perpetual motion machine; evaporation; turbine engine; solar energy; photovoltaics; Googleplex; megawatt; Las Vegas; molten salt; California Institute of Technology; Pasadena, CA; cerium oxide; chemical element; exothermic reaction; partial pressure; oxygen; Gibbs free energy of formation; Gnumeric; reduction; water; carbon dioxide; hydrogen; carbon monoxide; syngas; hydrocarbon; catalyst; methane; carbon sequestration; fossil fuel plant; carbon; inert atmosphere.