The 2011 Nobel Prize in Chemistry
October 7, 2011
The lines between
scientific disciplines are not that distinct. At least on paper, I'm a
materials scientist, but I've coauthored papers with
physicists,
electrical engineers and
chemists. There's a general scientific corpus that you need to learn to become skilled in any advanced scientific field. That's why we all take many of the same courses as
undergraduates. We have the example of
Marie Curie, who was awarded the
Nobel Prize in Physics in 1903 (for
radioactivity), and the
Nobel Prize in Chemistry in 1911 (for the discovery of
radium and
polonium).
This year's Nobel Prize in Chemistry could just have been awarded in physics. In fact, its
crystallographic background was the subject of the award of the 1914 Nobel Prize in Physics to
Max von Laue "for his discovery of the
diffraction of X-rays by
crystals," and the 1915 Nobel Prize in Physics to the father-son team of
William Henry Bragg and
William Lawrence Bragg for the "analysis of crystal structure by means of X-rays."
The 2011 Nobel Prize in Chemistry was awarded to the
Israeli,
Dan Shechtman, "for the discovery of
quasicrystals."[1-7] A quasicrystal is a material that exhibits some of the properties of a crystal, such as
long-range order, but its structure does not have
translational periodicity. The reason that such subject matter is Nobel Prize material is that, before Shechtman's 1984 publication on his discovery, such an idea was absurd.[8] Shechtman, as sole recipient, gets the entire $1.5 million monetary award.
Although his publication was in 1984, the actual discovery was made in 1982 (see figure). It took two years for Shechtman to get his paper accepted for publication.[3]
Linus Pauling, who was generally open-minded about new ideas, didn't believe that such crystals could exist, and he searched for methods by which
crystallographic defects could give the same result.[2]
Shechtman was finally able to get his work published by adding a corp of respectable coauthors: Ilan Blech, a materials scientist, Denis Gratias, a crystallographer, and
John Cahn, a physicist at
NIST.[8] Shechtman was working on a NIST project while on sabbatical at
Johns Hopkins University.[2] Said Cahn, "All I did was present the wonderful work that he had done in a compelling way."[3]
After publication, however, numerous other examples appeared, in alloys of
aluminum,
copper, and
iron; and of
ytterbium and
cadmium.[7] There was even one natural source, an alloy of aluminium, copper and iron supposedly excavated from rocks in the
Koryak Mountains in
Russia.[3] The name, quasicrystal, was used by other authors, and the name stuck.[9]
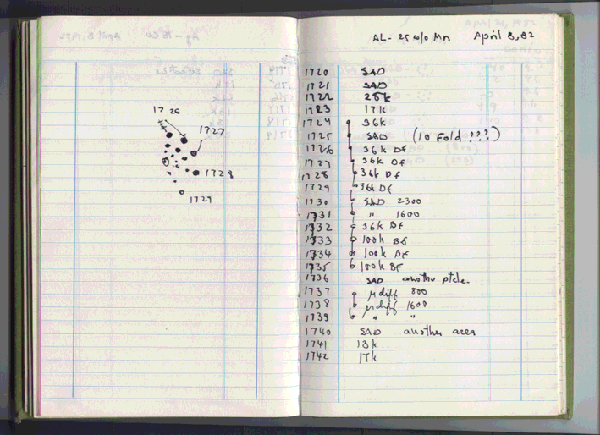
The page in Dan Shechtman's laboratory notebook noting his April 8, 1982, discovery of a quasicrystal. Whenever you see multiple exclamation points in a scientist's laboratory notebook, it's either very good news, or some equipment problem. (Via Iowa State University).
Shechtman's discovery involved an alloy of aluminum that contained fourteen atomic percent
manganese.[8] The alloy was prepared by
rapid solidification, a technique that inhibits motion of atoms as the material solidifies. What Shechtman found using
electron diffraction was concentric diffraction circles of ten equally-spaced dots; that is, the material exhibited ten-fold
rotational symmetry.[6-7]
The spots were as sharp as for any crystal, but the diffraction indicated an
icosahedral point group symmetry. This meant that the lattice was not uniform under translation, and the diffraction couldn't be indexed to any
Bravais lattice.[8] Crystallography forbids 5-fold (and 10-fold) symmetry.
There was some background to the discovery.
Johannes Kepler, who was forever searching for order in the
universe, presented quasicrystal-like patterns in his book
Mysterium Cosmographicum.[4] More recently, the
mathematical physicist,
Roger Penrose, created
aperiodic tiling patterns (see figure).[4] Penrose asked Shechtman if these tilings had any influence on his work. Shechtman responded that, although he knew about them, they didn't come to mind during the time of his discovery.[4]
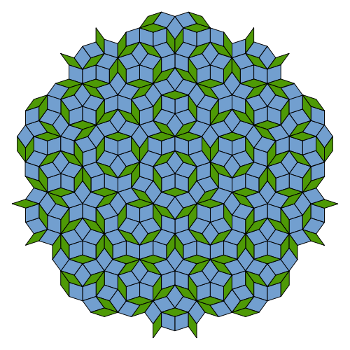
A Penrose tiling (P3) using thick and thin rhombi. The symmetry is five-fold, and the structure is aperiodic.
(Via Wikimedia Commons))
One curious thing about quasicrystals is the fact that the ratio of various distances between atoms involves the
golden mean.[1] Aside from that tantalizing clue, it's not known why atoms will arrange themselves into quasicrystalline structures. The structures, however, do exhibit some unusual properties, probably because the contained
electrons are confused. Actually, the scientific term would be "
frustrated." These attributes might apply only to
anthropomorphic electrons.
Quasicrystals formed from metals are non-metallic. They are poor
thermal and
electrical conductors, their surfaces exhibit low
friction, they don't
oxidize, and they are
hard.[3-4] These useful properties might not be obtained in practical applications, since these materials are
metastable; that is, they will revert to a normal crystalline form when heated.
This was
paradigm-busting work, so much so that in 1992 the
International Union of Crystallography changed its definition of a crystal from "a regularly ordered, repeating three-dimensional pattern" to a solid with a "discrete diffraction diagram."[2]
References:
- The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry for 2011 to Dan Shechtman, Press Release, October 5, 2011.
- Kenneth Chan, "Israeli Scientist Wins Nobel Prize for Chemistry," The New York Times, October 5, 2011.
- Richard Van Noorden, "Impossible crystals snag chemistry Nobel," Nature, October 5, 2011.
- Ian Sample, "Nobel Prize in Chemistry for dogged work on 'impossible' quasicrystals," Guardian (UK), October 5, 2011.
- Ian Sample, "Nobel Prize in Chemistry 2011 – live blog,"This year's Nobel Prize in Chemistry has been won by Daniel Shechtman for the discovery of quasicrystals," Guardian (UK), October 5, 2011.
- Andrea Gerlin, "Shechtman Wins Chemistry Nobel for Crystal Find," Bloomberg, October 5, 2011.
- Mitch Jacoby, "Nobel Prize In Chemistry - Dan Shechtman wins for discovering convention-bucking quasicrystals," Chemical & Engineering News, October 5, 2011.
- D. Shechtman, I. Blech, D. Gratias and J.W. Cahn, "Metallic phase with long range orientational order and no translation symmetry,", Physical Review Letters, vol. 53, no. 20 (November 12, 1984), pp. 1951-1953.
- D. Levine and R. Steinhardt, "Quasicrystals: a new class of ordered structures," Physical Review Letters, vol. 53, no. 26 (December 24, 1984), pp. 2477-2480.
- Daniel Shechtman web page at Technion.
Permanent Link to this article
Linked Keywords: Scientific; materials scientist; physicist; electrical engineer; chemist; undergraduate; Marie Curie; Nobel Prize in Physics; radioactivity; Nobel Prize in Chemistry; radium; polonium; crystallographic; Max von Laue; X-ray crystallography; diffraction of X-rays; crystal; William Henry Bragg; William Lawrence Bragg; Israeli; Dan Shechtman; quasicrystal; long-range order; translational periodicity; Linus Pauling; crystallographic defect; John Cahn; National Institute of Standards and Technology; NIST; Johns Hopkins University; aluminum; copper; iron; ytterbium; cadmium; Koryak Mountains; Russia; laboratory notebook; Iowa State University; manganese; rapid solidification; electron diffraction; rotational symmetry; icosahedral symmetry; icosahedral point group symmetry; Bravais lattice; Johannes Kepler; universe; Mysterium Cosmographicum; mathematical physicist; Roger Penrose; Penrose tiling; aperiodic tiling pattern; Wikimedia Commons; golden mean; electron; frustrated; anthropomorphic; thermal conductors; electrical conductors; friction; rust; oxidation; hardness; metastable; paradigm; International Union of Crystallography.