
Mica
February 5, 2024 Micas are a group of phyllosilicate minerals with perfect basal cleavage. This allows their being easily split into very thin elastic sheets. The most common mica is Muscovite, also known as eisenglass ("iron glass"). The lyrics of The Surrey with the Fringe on Top, a song from the 1943 musical, Oklahoma! by Richard Rodgers (1902-1979) and Oscar Hammerstein (1895-1960), mentions an isinglass curtain; but the material would have been mica.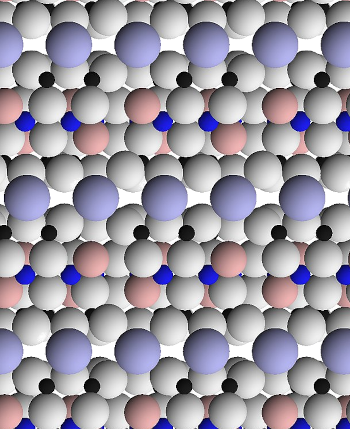
This atomic representation of Muscovite mica shows a view parallel to its layers. Two full layers are seen at the image center.
Muscovite has the general formula (KF)2(Al2O3)3(SiO2)6(H2O).
Oxygen anions are white, fluoride anions are light pink, tetrahedral silicon or aluminum cations are black, octahedral aluminum cations are dark blue, and potassium cations are light blue. The layers are bound together by potassium cations.
(Wikimedia Commons image by Kent G. Budge. Click for larger image.)
Mica was a common electronic material in the early 20th century because of its availability as large sheets, often many square meters in area, that could be cut, die-stamped, and machined to close tolerance. Mica was used as the dielectric material in stable, high-Q silver mica capacitors in radio transmitters and radio receivers. Mica sheets also provided support for heating wires up to 900 °C (1,650 °F) in heat guns and other heating devices.
A common application for mica in late 20th century electronics.
These thin sheets provided good thermal conductivity for heat transfer from a power transistor to a heat sink with electrical insulation.
Wikimedia Commons
As I've stated in many previous articles, science advances more from the development of improved instrumentation than through theory. The most recently developed instruments for surface studies, the scanning tunneling microscope (1981) and the atomic force microscope (1985), were developed by the talented engineers and physicists, Gerd Binnig (b. 1947), Heinrich Rohrer (1933-2013), Calvin Quate (1923-2019), and Christoph Gerber (b. 1942). A research team from the Vienna University of Technology (TU Wien, Vienna, Austria) and Charles University (Prague, Czech Republic) has used atomic force microscopy (AFM) to determine the distribution of K+ cations at low temperatures on cleaved mica under ultra-high vacuum.[1-2] Their research is described in an open access article in Nature Communications.[1] Surfaces of atomically thin materials, such as graphene and molybdenum disulfide, are a popular research area in solid state physics since they have properties that are different from their bulk materials.[2] Mica has been an object of study in many diverse fields, including biochemistry, geochemistry, nanotribology, and electronics.[1] However, the surface structure of mica has not been studied on an atomic scale.[2] The mica crystal structure allows for incorporation of many elements, and Muscovite mica has a layered structure of alternating aluminosilicate and K+ layers.[1] Muscovite splits apart at the K+ layers, and there are an equal number of such cations on each created surface so that charge neutrality can be maintained.[1] The present study is an attempt to find whether K+ cations are distributed randomly on the surface, ow whether they were ordered.[1] The mica surface, as are all surfaces, is difficult to examine, since atoms and molecules from the environment are easily adsorbed. The Vienna University of Technology is fortunate in having developed an atomic force microscope that images materials in an ultra-high vacuum.[2] Says post-doctoral researcher and first author of the paper, Giada Franceschi,
"We were able to see how the potassium ions are distributed on the surface... We were also able to gain insights into the positions of the aluminum ions under the surface layer - this is a particularly difficult task experimentally."[2]
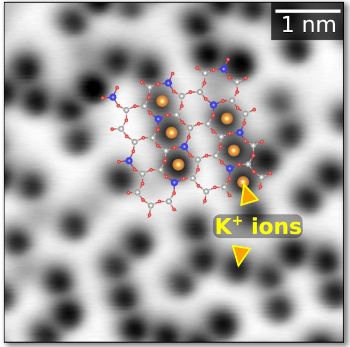
Not so random.
The study shows that the K+ cations on the surface, imaged as black, are arranged with short-range order.
The images were acquired using a stiff AFM cantilever that is less affected by the highly-charged surface of mica that will reduce atomic contrast.
The K+ cations sit on an hexagonal lattice of lattice constant 0.52 nm and occupy approximately half of the surface sites.[1]
(Vienna University of Technology image. Click for larger image.)
The AFM images showed that the K+ cations are not randomly distributed on the surface, but are grouped into small assemblages (see figure), a condition of short-range order.[1-2] The possibility of this order was confirmed by density functional theory (DFT) calculations and Monte Carlo simulations that reveal the importance of the subsurface Al3+ cations to the surface K+ cation arrangement.[1] This reveals a potential problem in the use of mica as an insulating substrate for graphene electronics.[2]
References:
- Giada Franceschi, Pavel Kocán, Andrea Conti, Sebastian Brandstetter, Jan Balajka, Igor Sokolović, Markus Valtiner, Florian Mittendorfer, Michael Schmid, Martin Setvín, and Ulrike Diebold, "Resolving the intrinsic short-range ordering of K+ ions on cleaved muscovite mica," Nature Communications, vol. 14, no. 1 (January 13, 2023), Article no. 208, https://doi.org/10.1038/s41467-023-35872-y. This is an open access article with a PDF file here.
- The Last Mysteries of Mica, Vienna University of Technology Press Release, January 25, 2023.