
Critical Materials
April 22, 2024 Human culture has been defined by materials to the extent that archaeologists have named some periods of human activity by their premier materials. In the so-called three-age system, we've lived in the Stone Age, which ended about 4000 BC; the Bronze Age, which started around 2500 BC, when metalworking was first practiced; and the Iron Age, which started around 1000 BC. The proposed Anthropocene, the present age of man, could be defined by increased trace elements, notably radionuclides, in the geological record. The mass of synthetic materials now outweighs all Earth's biomass.[1] Since gold is the least reactive metal, it occurs in nature as the metal, itself, so it was the first metal used by man. After gold, primitive man discovered ways to release other metals from their ores by heating. The ancients were able to smelt silver, mercury, copper, lead, tin, and finally, iron. Bronze, an alloy of copper and tin, was a stronger metal than copper, and it had sufficient properties to launch the Bronze Age.
Hesiod, Works and Days, lines 150-151, scanned from the author's copy of ref. 2. The translation is "Their armor was of bronze, and their houses of bronze, and of bronze were their implements: there was no black iron."[2]
Iron smelting, the extraction of iron from its common ores, is much more difficult than the extraction of the other metals since it requires higher temperature. The reduction of the common iron ore, hematite (Fe2O3), using carbon as a reducing agent is straightforward; viz.,
2Fe2O3 + 3C -> 4Fe + 3CO2This so-called carbothermic reaction uses readily available materials, but it happens at higher temperatures than encountered for the smelting of the other metals known to the ancients. These temperatures were achieved after development of bloomery furnaces.
 The 150th anniversary of the Periodic Table was in 2019, proclaimed the International Year of the Periodic Table of Chemical Elements (IYPT2019) by the United Nations General Assembly. Russian chemist, Dmitri Mendeleev (1834-1907), organized all the chemical elements known in 1869 into a periodic table based on atomic weight.[3]
The 150th anniversary of the Periodic Table was in 2019, proclaimed the International Year of the Periodic Table of Chemical Elements (IYPT2019) by the United Nations General Assembly. Russian chemist, Dmitri Mendeleev (1834-1907), organized all the chemical elements known in 1869 into a periodic table based on atomic weight.[3]
(Wikimedia Commons image of Dmitri Mendeleev in 1887, modified for artistic effect.)
Fewer than half of the 118 elements of today's periodic table were known to Mendeleev. The present plethora of 118 elements has given scientists a huge palette of materials with which to create compounds of technological importance. Most prominent among these are alloys for high strength permanent magnets, lithium cathodes for rechargeable batteries, and high temperature alloys for gas turbine engines and turbine generators. The essential problem is extraction of large quantities of these sometimes trace elements from the Earth's crust. The uneven distribution of needed elements adds geopolitics to the extraction problem. Notably, China produces 60 percent of the rare earth elements needed for such things as NdFeB magnets used in motors and generators, phosphors and catalysts.[4] China also imports rare earth elements from other countries to process nearly 90 percent of worldwide rare earths into end products.[4] As shown in the following table, lithium is likewise concentrated in just a few countries.
| Country | Metric Tons | Percent |
|---|---|---|
| Australia | 55,416 | 52 |
| Chile | 26,000 | 25 |
| China | 14,000 | 13 |
| Argentina | 5,967 | 6 |
| Brazil | 1,500 | 1 |
| Zimbabwe | 1,200 | 1 |
| Portugal | 900 | 1 |
| United States | 900 | 1 |
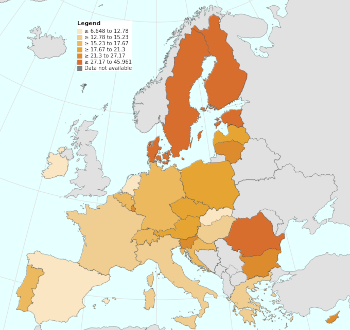 European Union statistics show that Europeans consume 15 metric tons of materials per person annually.[8] The consumption ranges from 7 metric tons per person for The Netherlands to Finland's 46 (see figure).[8]
European Union statistics show that Europeans consume 15 metric tons of materials per person annually.[8] The consumption ranges from 7 metric tons per person for The Netherlands to Finland's 46 (see figure).[8]Finland also generates the most per capita waste (20,993 kg), about seven times that of the least wasteful country, Croatia (1,483 kg).[8] The average waste generation of a European Union citizen in 2020 was 4,815 kg.[8]
(Map from the European Union Eurostat showing the annual raw material consumption. Click for larger image.)
References:
- Emily Elhacham, Liad Ben-Uri, Jonathan Grozovski, Yinon M. Bar-On, and Ron Milo, "Global human-made mass exceeds all living biomass," Nature. vol. 588, no. 7838 (December 9, 2020), pp. 442-444, https://doi.org/10.1038/s41586-020-3010-5.
- Hugh G. Evelyn-White, Trans., "Hesiod, The Homeric Hymns and Homerica," William Heinemann/The Macmillan Co., London/New York, 1914, pp. 12-13 (via the Internet Archive).
- Dmitri Mendeleev, "Die periodische Gesetzmäßigkeit der Elemente," Annalen der Chemie und Pharmacie, vol. VIII. Supplement (1871), pp. 133-229. An image of Mendeleev's 1871 periodic table can be found at Wikimedia Commons, here.
- Gracelin Baskaran, "What China’s Ban on Rare Earths Processing Technology Exports Means," Center for Strategic and International Studies, January 8, 2024.
- Govind Bhutada, "This chart shows which countries produce the most lithium," World Economic Forum, January 5, 2023.
- What Are Critical Materials and Critical Minerals?, Critical Minerals & Materials Program, United States Department of Energy.
- Diana J. Bauer, Ruby T. Nguyen, Braeton J. Smith, et al., "Critical Materials Assessment," U.S. Department of Energy, July 2023
- Arthur Neslen, "Extraction of raw materials to rise by 60% by 2060, says UN report," The Guardian (UK), January 31, 2024.
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Critical Raw Materials Resilience: Charting a Path Towards Greater Security And Sustainability, Document 52020DC0474, EUR-Lex, Publications Office of the European Union.