
Hygroscopic Energy
March 6, 2023 Battery power has enhanced our quality of life by energizing small devices, such as smartphones and tablet computers, as well as our much larger electric cars. Many of these devices incorporate rechargeable batteries, but there are still many devices, such as television remote controls and children's toys, that utilize non-rechargeable primary batteries enabled by irreversible electrochemical reactions. A simple example of a primary battery is the copper/zinc cell. The first battery, the 1799 voltaic pile of Alessandro Volta (1745-1827), was a series arrangement of copper/zinc cells using salt water brine as the electrolyte. A modern example, as shown in the figure, uses a sulfuric acid electrolyte. Its irreversibility is easily seen by the loss of hydrogen gas from the system.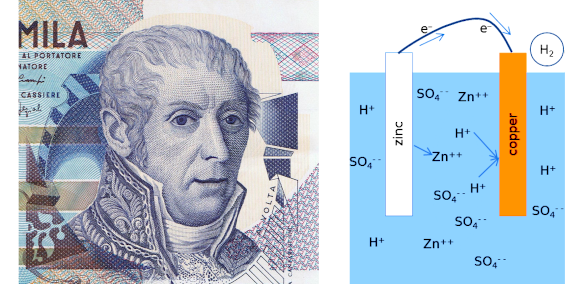
Alessandro Volta (1745-1827) and a copper/zinc battery. The zinc anode (the material that supplies the electrons to an external circuit) and copper cathode (the material accepting electrons from an external circuit) are immersed in a sulfuric acid electrolyte. Doubly-charged zinc ions are dissolved in the electrolyte and hydrogen ions combine with electrons at the copper cathode to produce hydrogen gas. (Left image, portion of a Wikimedia Commons image of a 10,000 Italian Lira bank note. Right image, a Wikimedia Commons image by Easchiff. Click for larger image.)
Although batteries operate by chemical reactions, the real stars of the process are the ions and electrons. You can create a battery without a chemical reaction if you have a reservoir of ions and can move them from one electrode to another. This approach was used in a device that produces electric power from humid air.[1-3] The research team members were from the National University of Singapore, the Agency for Science, Technology and Research (Singapore), and Bruker Corporation (Singapore). The team was led by Tan Swee Ching, an assistant professor in the Department of Materials Science and Engineering of the National University of Singapore.[2] Presently, such devices known as moisture-driven energy generators (MEG) will produce energy only until they are saturated with water.[1-2] The Singapore research team developed a novel asymmetric hygroscopic structure that harvests energy from moisture in the air and stores the generated electricity as a battery would.[1] The device consists of a 0.3 millimeter thick layer of a non-woven fabric of wood pulp and polyester coated with carbon nanoparticles.[1-3] One side of a strip of fabric, the wet region, is coated with a hygroscopic ionic hydrogel that absorbs about 4.5 times its weight in water.[3] Sea salt, mixed into the hydrogel, is used to harvest moisture from the air.[2] Says Tan,
"Sea salt was chosen as the water-absorbing compound due to its non-toxic properties and its potential to provide a sustainable option for desalination plants to dispose of the generated sea salt and brine."[2]The other side of the fabric strip, the dry region, does not contain the hygroscopic ionic hydrogel layer.[2] Absorbed water from the air creates a wet-dry asymmetry across the strip and this produces an in-plane electric field that exists even after saturated water absorption.[1] The absorbed water creates an electrical double layer, as in supercapacitor, over the carbon surface.[1] The supercapacitor structure allows for energy storage.[1] A single strip produces a potential of nearly a volt, and the strips can be connected in series for larger voltage, as in that of conventional batteries.[3]
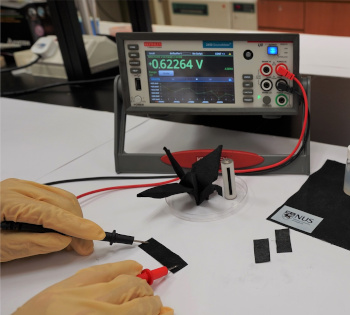
Voltage output of a moisture-driven energy generator strip. The digital voltmeter reads about 0.62 volts.
The carbon coating causes the black color of the strip. An origami folded strip, still capable of voltage generation, can be seen in front of the voltmeter.
(Portion of a National University of Singapore image, also here.)
After being saturated by water, a 1.5 centimeter x 2 centimeter strip was shown to provide a voltage of about 0.7 volts for more than 150 hours.[2] After 30 days, the hydrogel was still saturated with water, the voltage output was the same, and a peak power density of 70 microwatts per cm3 was realized.[1] The device can store electricity, just as a supercapacitor, when voltage is applied.[1-2] This energy harvester has high flexibility and it can withstand stress from twisting, rolling, and bending.[2] As a demonstration of this, an origami crane was created with the fabric, and the overall electrical performance of the device was not affected.[2] One such strip will output 0.7 volts, but a series connection of strips will give higher voltage, such as the 1.5-1.9 volts of a typical battery[3] As a demonstration of this, the researchers integrated strips into a 3D printed case that was the size of a standard AA battery.[2] The voltage of that battery was as high as 1.96 volts, and it powered the analog alarm clock shown below.[2-3]

Analog alarm clock powered by a battery created from moisture-driven energy generating strips. (Still images from a YouTube video by the National University of Singapore College of Design and Engineering.)[3]
The raw materials for this device are easily obtained, and the final fabricated cost is about ten cents per square meter.[2] As Tan says, "Our device shows excellent scalability at a low fabrication cost. Compared to other MEG structures and devices, our invention is simpler and easier for scaling-up integrations and connections. We believe it holds vast promise for commercialization."[2] The researchers have filed a patent for this technology.[2]
References:
- Yaoxin Zhang, Shuai Guo, Zhi Gen Yu, Hao Qu, Wanxin Sun, Jiachen Yang, Lakshmi Suresh, Xueping Zhang, J. Justin Koh, and Swee Ching Tan, "An Asymmetric Hygroscopic Structure for Moisture-Driven Hygro-Ionic Electricity Generation and Storage," Advanced Materials, vol. 34, no. 21 (May 26, 2022), article no. 2201228, DOI: https://doi.org/10.1002/adma.202201228.
- NUS researchers invent self-charging, ultra-thin device that generates electricity from air moisture, National University of Singapore Press Release, August 17, 2022.
- A battery powered by moisture from the air, YouTube Video by the National University of Singapore College of Design and Engineering.