
Cement Supercapacitors
October 9, 2023 Most scientific research is done with a limited budget. As a consequence, experimenters try to use inexpensive materials whenever possible. Early in the 20th century, Ernest Rutherford and his colleagues were making fundamental discoveries in particle physics using string and sealing wax. As Richard Reeves wrote in his biography of Rutherford, when one of Rutherford's students needed a metal tube for an experiment, Rutherford got it by cutting a piece from the handlebar of an old bicycle.[1] India ink is a type of carbon black ink that's a colloid of fine carbon soot particles in aqueous solution. Carbon black ink was often used in physics experiments as an electrical conductor, since the carbon particles join together upon removal of the water. The carbon particles form a conductive percolation network when the ink dries, and the India ink can be used for connecting wires to material specimens that can't be soldered. Carbon pigment ink has been used for millennia, and it was used as the ink for the Dead Sea Scrolls (see figure)
Portion of the Temple Scroll, one of the Dead Sea Scrolls.
The Dead Sea Scrolls have had an interesting history that includes a novel use of an early desktop computer.
Although discovered in 1947, a small group of scholars withed their contents through 1990.
Finally, one scholar typed a concordance of the scrolls into a Macintosh computer and used a computer program to reconstruct the text from these data.[2]
(Wikimedia Commons image of the Temple Scroll at the Israel Museum. Click for larger image.)
A research team from the Massachusetts Institute of Technology (Cambridge, Massachusetts) and Harvard University (Cambridge, Massachusetts), has combined India ink with another inexpensive material, cement, to create supercapacitors.[3-6] The research team was led by MIT professors, Franz-Josef Ulm, Admir Masic, and Yang Shao-Horn.[8] This research follows from MIT's previous investigation in which these materials were used to create conductive cement.[7-8] The conductive cement allowed Joule heating (resistive heating) in which the cement reached a temperature of 41°C (about 100°F) in samples a few cm3 in size.[8] Potential uses for conductive cement are as a deicer replacement for environmentally harmful salts and other chemicals for roadways and airport runways.[8]
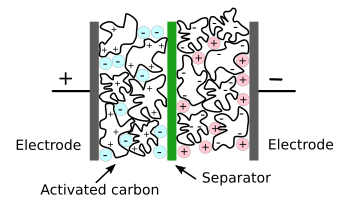 In an electrochemical double-layer capacitor (supercapacitor), application of a voltage to the electrodes causes cations to move to the negative electrode, and anionanions to move to the positive electrode. The separator isolates the two halves of the capacitor. Discharge through circuitry connected between the electrodes recovers the energy stored by charging.
In an electrochemical double-layer capacitor (supercapacitor), application of a voltage to the electrodes causes cations to move to the negative electrode, and anionanions to move to the positive electrode. The separator isolates the two halves of the capacitor. Discharge through circuitry connected between the electrodes recovers the energy stored by charging.(Modified Wikimedia Commons image by Stan Zurek. Click for larger image.)
Renewable energy sources such as solar and wind power are sporadic, and they require energy storage for reliability. Unfortunately, existing battery technologies are expensive, and they rely on scarce materials such as lithium.[4] Energy storage in cement supercapacitors could become an inexpensive alternative.[3] The two plates of the capacitor act as if they were the two poles of a rechargeable battery.[4] The essential requirement for a useful supercapacitor is a large surface area of its conductive plates.[4] The carbon black network embedded within the cement fulfills this requirement.[4] The hydration reactions of cement cause the carbon black particles form a fractal-like conductive network.[3] This is accomplished by addition of water in excess of the stoichiometric water limit, usually 42% by mass of cement.[3] The water forms a branching network of openings as it a reacts with cement, and the carbon particles move into these spaces since they are hydrophobic, forming the fractal-like conductive network with an extremely large surface area.[4] The cement-carbon black composite is then soaked in an electrolyte, such as potassium chloride, which provides the anions and cations that accumulate on the carbon conductor.[4] Two disks of this material are separated by an insulating membrane to form the supercapacitor.[4] Carbon black addition of merely 3 percent by volume will achieve a conductive network.[4] Says study co-author, Franz-Josef Ulm, a professor of Civil and Environmental Engineering at MIT,
"What we found is that the maximum energy storage capacity only depends on the specific surface of the carbon black... Since this carbon black is space-filling—thanks to the magic of chemistry, we can simply increase the volume to create large-scale bulk energy solutions."[6]The research team demonstrated the process by creating supercapacitors about 1 centimeter across and 1 millimeter thick, about the size of most button cell batteries,[4] These could be charged to 1 volt, and three in series could energize a light-emitting diode (LED).[4,6] The storage capacity remained constant after 10,000 charge-discharge cycles.[6] Adding carbon black beyond 10 percent by volume increased the storage capacity of the supercapacitor, but carbon reduces the strength of the cement.[6] Ten percent carbon black provided good charge capacity at reasonable strength.[4]
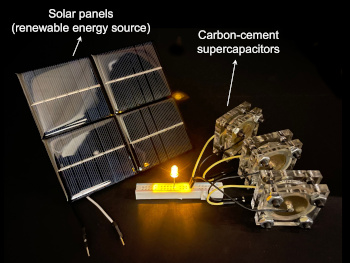
MIT cement supercapacitors exciting a light-emitting diode/a>. Also shown is a solar power panel as an example of a renewable energy source.
A supercapacitor cell is a low voltage device; so, several in series are needed in most power supply applications.
(MIT image, released under a Creative Commons license.)
One application of this technology is using the cement-carbon black composite as the concrete foundation of a house. A 45 cubic meter block is calculated to have enough capacity to store about 10 kilowatt-hours of energy, about 0ne third the average daily electricity usage for a household.[4-6] The research team has patented this technology and now plans to build a series of larger versions, starting with ones about the size of a typical 12-volt car battery, then working up to a 45-cubic meter demonstration unit with the ability to store household power.[4-5]
References:
- Richard Reeves, "A Force of Nature: The Frontier Genius of Ernest Rutherford," W. W. Norton, December 3, 2007, 208 pp. (via Amazon).
- Ron Grossman, "Computer Generates Bootleg Copy Of Dead Sea Scrolls -- Official Committee Has Refused To Publish Its Translations Of Texts," Seattle Times, September 4, 1991.
- Nicolas Chanut, Damian Stefaniuk, James C. Weaver, Yunguang Zhu, Yang Shao-Horn, Admir Masic, Franz-Josef Ulm, "Carbon–cement supercapacitors as a scalable bulk energy storage solution," Proc. Natl. Acad. Sci., vol. 120, no. 32 )July 31, 2023), Article no. e2304318120, https://doi.org/10.1073/pnas.2304318120. This is an open access article with a link to a PDF file at this same URL.
- David L. Chandler, "MIT engineers create an energy-storing supercapacitor from ancient materials," MIT Press Release, July 31, 2023.
- Robert F. Service, "Electrified cement could turn houses and roads into nearly limitless batteries," Science, July 31, 2023, doi: 10.1126/science.adk0583.
- Sarah Wells, "This Supercapacitor Is Made From Cement," IEEE Spectrum, August 3, 2023.
- Nancy A. Soliman, Nicolas Chanut, Vincent Deman, Zoe Lallas, and Franz-Josef Ulm, "Electric energy dissipation and electric tortuosity in electron conductive cement-based materials," Phys. Rev. Materials, vol. 4, Article no, 125401, December 9, 2020, DOI:https://doi.org/10.1103/PhysRevMaterials.4.125401.
- Andrew Logan, "Electrifying cement with nanocarbon black, MIT Press Release, April 20, 2021.