
Adhesives
December 4, 2023 Most experiments involve disparate components that need to be combined. Physicists through the early 20th century used sealing wax as an instant glue. One of the most important items in my laboratory is two-part five-minute epoxy, often in a high viscosity putty form. I've also employed UV-curable photopolymers that are now much easier to use after the invention of UV-emitting LEDs. I sometimes use a cyanoacrylate adhesive, also known as superglue.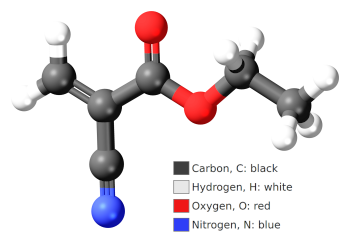
Ball and stick model of ethyl cyanoacrylate C6H7NO2, a common precursor to cyanoacrylate adhesive, also known as superglue.
(Modified Wikimedia Commons image by Jynto. Click for larger image.)
The first cyanoacrylate polymer was patented in 1949 by the B.F. Goodrich Company.[1] Its research team was looking for a transparent plastic material that could be used in optics; but, as frequently happens in science, its adhesive property was an accidental result. Their formulation had the undesirable property that it stuck to everything with which it came in contact. In 1951, Harry Coover Jr. (1917-2011) and Fred Joyner continued research on cyanoacrylate polymers at Eastman Kodak, and Kodak eventually marketed its famous Eastman 910 adhesive in 1958. Superglue is not entirely super, since glued components can be separated in mechanical shear. Superglue also has caused problems when used with optical components, since excess cyanoacrylate monomer will vaporize, polymerize upon contact with moisture in the air, and deposit on lenses and filters to reduce transparency and increase light scattering. I dimly remember some reports that stress induced by the adhesive has caused surface cracks on optical components. As anyone who's wrestled with the installation a natural fir Christmas tree knows, pine sap is extremely sticky. Canada balsam cement, the oleoresin extracted from the balsam fir (abies balsamea), was used in the past to glue optical components together. It's transparent when dry, and has a refractive index of 1.55 that matches that of crown glass. Today's synthetic epoxies have replaced Canada balsam cement.

European mistletoe (Viscum album) berries found at Wrocław, Poland.
(Wikimedia Commons image by Agnieszka Kwiecień. Click for larger image.)
As I wrote in an earlier article (Mistletoe Glue, August 15, 2022), mistletoe berries have a coating of viscin, a natural adhesive consisting of hierarchically organized cellulose microfibrils and mucopolysaccharides. European mistletoe (Viscum album) is a parasitic plant species. It was used for medicinal purposes at the time of the ancient Greeks, and possibly earlier.[2] Mistletoe's fiber-reinforced adhesive has an evolutionary advantage for seed dispersal of this parasitic plant, since it allows the mistletoe seeds to stick to and infest host plants.[2] Adhesives are not the only thing that can bond objects together. Gauge blocks are ceramic or metal blocks that have been polished to extreme flatness and smoothness, and this polishing allows the blocks to be joined together with interatomic forces in contact. These blocks are known by machinists as Jo Blocks in honor of their originator, Swedish inventor, Carl Edvard Johansson (1864-1943), who proved this idea in his home laboratory. After further development, Johansson was granted a Swedish patent entitled, "Gauge Block Sets for Precision Measurement," in 1901. He started his own company in 1917, moved the company to the United States, and sold the company to Ford in 1923 to form its Johansson division. He was posthumously awarded a gold medal by the Royal Swedish Academy of Engineering Sciences in 1943. The mechanism for bonding with adhesives is similar in principle to the interatomic bonding between surfaces in gauge blocks. The adhesive wets the opposing rough surfaces and flows onto and into the surface texture.[3] This allows interatomic bonding between the adhesive material and the rough surfaces. There's quite a bit of materials science involved in balancing the opposing requirements of adequate flow and mechanical strength. Often, adhesive tape is used as a way to temporarily affix something with the idea that it will be removed after it's served its purpose. One painful reminder of this are adhesive bandages (band-aids), whose adhesive strength must be strong enough to bond to skin for a day, or two; but, this leads to difficult removal. A recent paper in Nature Materials by mechanical engineers and scientists from Virginia Tech (Blacksburg, Virginia), the University of Colorado (Boulder, Colorado), Iowa State University (Ames, Iowa), and the University of Nebraska - Lincoln (Lincoln, Nebraska) describe a technique that combines strong tape adhesion with easy removal.[4-5] The method uses U-shaped cuts in the tape that allow easy removal when pulled in one direction.[4-5]
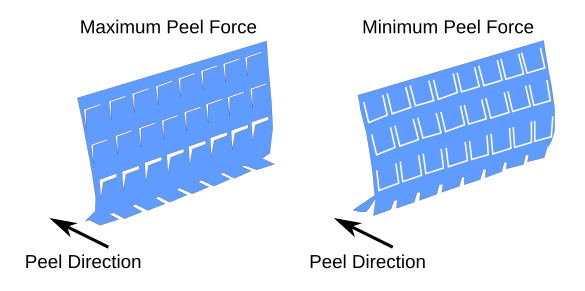
Illustration of peeling in each direction. It's easy to understand how the flap structure changes the peel strength. On the right, the peeling action is as if the flaps are not present, the adhesive tape acts as a continuous sheet, and removal is mostly by shear force. On the left, the flaps must be pulled from the surface in a direction opposite to the peeling direction. It's as if you're trying to pull the adhesive tape using a force normal to the sheet. (Created using Inkscape. Click for larger image.)
This adhesive tape is a metamaterial; that is, a material whose properties have been modified by geometrical structuring. The U-shaped cuts allow an easy delamination of the tape when peeled in one direction, and they suppress delamination when peeled in the other direction.[4-5] The researchers were easily able to create these metamaterial adhesive tapes with U-shaped flaps of a millimeter to a few centimeters dimension using a laser cutter.[5] The metamaterial tape was able to secure a picture frame to a wall 500 times longer (when the experiment was terminated) than the non-structured tape.[5] The upper strength of the adhesive can be controlled by changes in the dimension of the U-shaped flaps and their areal density.[5] Aside from its mundane use in adhesive bandages, this approach can be used for box-sealing tape, lightly adhesive gloves for fetching and releasing objects, and robotic grippers.[5]
References:
- Alan E Ardis, "Preparation of monomeric alkyl alpha-cyano-acrylates," US Patent No. 2,467,927, April 19, 1949.
- Nils Horbelt, Peter Fratzl, and Matthew J Harrington, "Mistletoe viscin: a hygro- and mechano-responsive cellulose-based adhesive for diverse material applications," PNAS Nexus, vol. 1, no. 1 (March 16, 2022), Article no. pgac026, pp. 1-11, https://doi.org/10.1093/pnasnexus/pgac026. This is an open access publication with a PDF file available at the article link.
- The Sciences - Ask The Experts, What exactly is the physical or chemical process that makes adhesive tape sticky?, Scientific American, July 14, 1997.
- Dohgyu Hwang, Chanhong Lee, Xingwei Yang, Jose M. Pérez-González, Jason Finnegan, Bernard Lee, Eric J. Markvicka, Rong Long, and Michael D. Bartlett, "Metamaterial adhesives for programmable adhesion through reverse crack propagation," Nature Materials, vol. 22 (June 22, 2023), pp. 1030-1038, https://doi.org/10.1038/s41563-023-01577-2.
- Sarah Wells, "Art-Inspired Tape Is Both Strong and Weak," Physics, vol. 16, no. 125 (July 18, 2023).