
Super-Tough CrCoNi Alloy
February 6, 2023 In the early 20th century, when graduation from high school was a terminal goal in education, people received much supplementary education from the cinema. Some detective movies had a dramatic scene in which a gemstone is tested to determine whether it's a genuine diamond by scratching a mirror. However, just as in today's Internet of false facts, it's a misconception that the only material capable of scratching glass is diamond itself. Quartz looks like diamond, and it has a Mohs hardness of 7, so it will scratch common glass with a Mohs hardness of about 5.5. Cubic zirconia, which is today's substitute for diamond in inexpensive jewelry, has a Mohs hardness of about 8.25, and it will easily scratch glass.
Early materials scientists: Theophrastus (c.371 - c.287 BC) (left) and Pliny the Elder (23-79 A.D.), also known as Pliny Secundus (Pliny the Second), on the right. Theophrastus is actually the nickname given to the Greek philosopher by his teacher, Aristotle (384 BC - 322 BC), and it translates to "divine style of expression." His treatise, On Stones, was a useful source for information on gems and minerals into the Renaissance. Theophrastus took a scientific approach by classifying rocks and gems based by their common properties.
Pliny's Naturalis Historia (Natural History, 77 AD) was essentially an encyclopedia of all ancient knowledge available to Pliny, and its importance is underscored by it's being one of the largest single works surviving from the Roman Empire. Pliny died in 79 AD during a rescue attempt of a friend and his family from the famous Mount Vesuvius eruption of that year.
(Left image, line engraving V0005785 from the Wellcome Trust, right image from an 1859 Milanese publication, both slightly modified, from Wikimedia Commons Click for larger image.)
Theophrastus in his treatise, On Stones, wrote that harder materials will scratch softer materials, and this fact was mentioned by Pliny the Elder in his Naturalis Historia (Natural History, 77 AD).[1-3] The scratch test of mineral hardness was placed on a scientific basis in 1812 by German mineralogist, Friedrich Mohs (1773-1839). Mohs created what's now called the Mohs scale of mineral hardness, an ordinal scale of minerals in which members of higher rank will scratch members of lower rank (see figure).
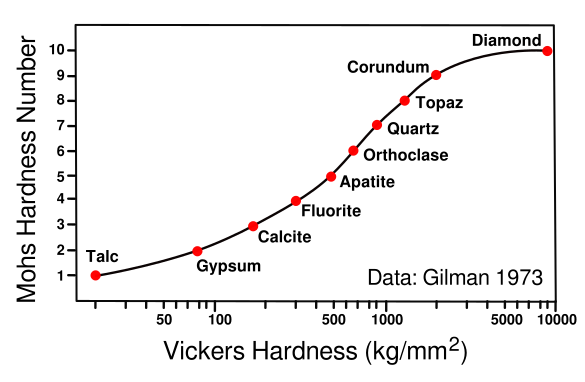
Relating hardness scales. The Vickers hardness test produces a material hardness number by pressing a pyrimidal indenter into the surface and measuring the dimensions of the indentation.
The graph shown relates Mohs hardness and Vickers hardness for the ten Mohs minerals, Talc, Mg3Si4O10(OH)2; Gypsum, CaSO4·2H2O; Calcite, CaCO3; Fluorite, CaF2; Apatite, Ca5(PO4)3(OH-,Cl-,F-); Orthoclase, KAlSi3O8; Quartz, SiO2; Topaz, Al2SiO4(OH-,F-)2; Corundum, Al2O3; and Diamond, C.
(Data from Gilman,[4] graphed using Inkscape. (Click for larger image.)
Diamond is at the top of Mohs hardness scale, but some materials might be harder than diamond. Diamond derives its strength from its short, directional covalent bonds between carbon atoms, and it's possible that similarly bonded compounds of low atomic number elements like boron, carbon, nitrogen, and oxygen will yield high hardness. As I wrote in an earlier article (Harder than Diamond, August 31, 2012), beta-carbon nitride (β-C3N4) was predicted in 1985 to have a hardness like diamond because it also has the same short and particularly incompressible covalent bonding between atoms. For that reason it became a target for synthesis, but there are presently no hardness data available for β-C3N4. The compound, rhenium diboride (ReB2) can be synthesized under ambient pressures, and the electronegativities of rhenium (1.9) and boron (2.04) are quite close, so their bonds have a mostly covalent character. A research team at UCLA prepared ReB2 and measured its microindentation hardness in 2007.[5-6] They found an average hardness of 48 GPa, whereas diamond has a hardness of more than 140 GPa. However, the UCLA team found that some of their specimens would scratch diamond, which indicates that ReB2, an hexagonal crystal, may be harder than diamond in some of its crystallographic directions. As an indicator of its incompressibility, the bulk modulus of ReB2 was found to be 360 GPa (diamond is 443 GPa). Materials are an important part of our lives; so, it's no wonder that authors have created fictional materials of extreme hardness to embellish their plots. Adamantium, sometimes called adamant or adamantine, is a fictional material of the same hardness as diamond. The comic book character, Wolverine, has a skeleton and claws strengthened by adamantium. The gates of hell, as depicted in John Milton's Paradise Lost and Virgil's Aeneid, are made of adamant. As Virgil writes,
Respicit Aeneas subito, et sub rupe sinistra moenia lata videt, triplici circumdata muro, quae rapidus flammis ambit torrentibus amnis, Tartareus Phlegethon, torquetque sonantia saxa. Porta adversa ingens, solidoque adamante columnae, vis ut nulla virum, non ipsi exscindere bello caelicolae valeant.[7]My favorite fictional material is Krell metal, the construction material of the ancient inhabitants of Altair IV, the fourth planet of the star Altair.[8] Krell metal is an important plot element of the film, Forbidden Planet.[8] Altair IV was once populated by the Krell, an extinct race that left behind many artifacts, including many made from Krell metal, a nearly indestructible alloy much stronger than adamantium.[8] I wrote about high-entropy alloys in a previous article (High-Entropy Alloys, June 20, 2016), These alloys are created from equal proportions of elements, so they differ from typical alloys, such as steel, that have a large matrix component modified by small additions of other elements. A significant property of high-entropy alloys is their reduced dislocation mobility that causes dislocations to grow more slowly. A recent article in Science reveals that the high-entropy alloy, CrCoNi, has outstanding damage tolerance.[9-12] It's being hailed as the material with the highest fracture toughness ever measured, and this fracture toughness extends to cryogenic temperatures.[9-12] High fracture resistance at very low temperatures is unusual, and it's important for spacecraft exposed to the cold of outer space. Research team members are from the University of Bristol (Bristol, UK), Lawrence Berkeley National Laboratory (Berkeley, California), Rutherford Appleton Laboratory (Oxon, UK), the University of California (Berkeley, California), the University of New South Wales (Sydney, Australia), Oak Ridge National Laboratory (Oak Ridge, Tennessee), the University of Tennessee (Knoxville, Tennessee), and Ruhr University (Bochum, Germany).[9] The research team was led by Robert Ritchie of Lawrence Berkeley National Laboratory and Easo George of Oak Ridge National Laboratory.[11] The decrease in fracture resistance at low temperatures was the apparent cause of the 1912 sinking of the Titanic in the frigid waters of the North Atlantic.[10] Most alloys show reduced fracture toughness at low temperatures, something that's especially acute at liquid helium temperature (4.15 K, -269°C;).[10] The research team investigated both CrCoNi and another low entropy alloy, CrMnFeCoNi, at 20 K.[9] CrMnFeCoNi did not perform as well as CrCoNi.[12]
Aeneas straightway by the leftward cliff beheld a spreading rampart, high begirt with triple wall, and circling round it ran a raging river of swift floods of flame, infernal Phlegethon, which whirls along loud-thundering rocks. A mighty gate is there columned in adamant; no human power, nor even the gods, against this gate prevail.[7]
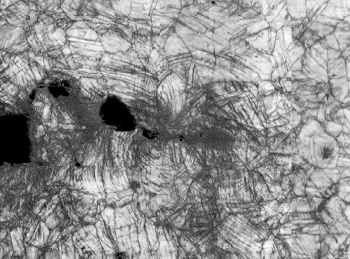
Microscope image of fracture in the CrCoNi alloy during stress testing at 20 kelvin (-424 °F).
The fracture is propagating from left to right.
(Robert Ritchie/Berkeley Lab image, also here. Click for larger image.)
Ritchie and George began experimenting with CrCoNi and CrMnFeCoNi about a decade ago, and they found impressive strength and toughness at liquid nitrogen temperature (77 K).[11] However, the instrumentation available to them was not capable of testing at temperatures lower than that, and it took a while to assemble a team with the necessary capability.[11] In the end, they were able to use such analytical tools as neutron diffraction, electron backscatter diffraction, and transmission electron microscopy to characterize such alloys at much lower temperature.[11] Dislocations are the determinant of both material ductility and fracture toughness. A greater number of dislocations gives a material more malleabiity, but material irregularities, such a grain boundaries, can block the dislocations from moving, giving increased strength but also decreased fracture toughness.[11-12] The researchers found that an unexpected phase transformation from face-centered cubic to hexagonal close packed in their high entropy alloys prevented the formation of cracks and their propagation.[9] This mechanism prolonged strain hardening to increase strength, ductility, and fracture toughness.[9,11] A multi-stage process occurs in which (1) portions of the crystal lattice slide away from each other such that the unit cells no longer match up, (2) applied force produces nanotwinning, (3) and increased force causes the cubic to hexagonal phase transition.[12]
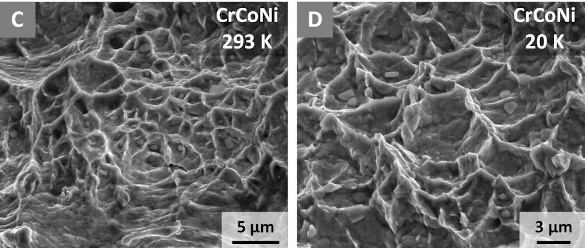
Electron backscatter diffraction images by a scanning electron microscope showing examples of fractures in CrCoNi at 293 K and 20 K. (Robert Ritchie/Berkeley Lab image, also here. Click for larger image.)
CrCoNi showed a fracture toughness of 459 megapascal-meters1/2, as high as 500 at 20 k, and CrMnFeCoNi showed 262 megapascal-meters1/2, and CrCoNi showed a fracture toughness exceeding 540 megapascal-meters1/2 after 2.25 millimeters of stable cracking.[9-12] In these same units, silicon is one, commercial aluminum airframe alloy is about 35, and the best steel is around 100.[11-12] While CrCoNi is expensive because of the cost of the component elements, the research team is investigating alloys formed from less expensive elements.[11] This research was funded by the U.S. Department of Energy and the U.K. Engineering and Physical Sciences Research Council, among other agencies.[9]
References:
- Theophrastus, On Stones, Greek Text, English Translation, and Commentary by Earle R. Caley and John F. C. Richards, 1956.
- Pliny the Elder: the Natural History, at Bill Thayer's University of Chicago Web Site.
- Pliny the Elder: the Natural History, Project Gutenberg.
- J. J. Gilman, "Hardness of pure alkali halides," Journal of Applied Physics, vol. 44, no. 3 (March, 1973), pp. 982ff., https://doi.org/10.1063/1.1662382.
- Hsiu-Ying Chung, Michelle B. Weinberger, Jonathan B. Levine, Abby Kavner, Jenn-Ming Yang, Sarah H. Tolbert, and Richard B. Kaner, "Synthesis of Ultra-Incompressible Superhard Rhenium Diboride at Ambient Pressure," Science, vol. 316. no. 5823 (April 20, 2007), pp. 436-439.
- Katharine Sanderson, "Scratching diamond just got easier: Ultra-hard material made in the lab without high pressures," Nature Online, April 19, 2007.
- P. Vergillius Maro, "Aeneid," book VI, ll. 548-554, via the Perseus Digital Library. Latin from J. B. Greenough, "Vergil. Bucolics, Aeneid, and Georgics," Ginn & Co. (Boston, 1900). English translation from Vergil, "Aeneid," Theodore C. Williams. trans., Houghton Mifflin Co. (Boston, 1910).
- Forbidden Planet (1956, Fred M. Wilcox, Director) on the Internet Movie Database.
- Dong Liu, Qin Yu, Saurabh Kabra, Ming Jiang, Paul Forna-Kreutzer, Ruopeng Zhang, Madelyn Payne, Flynn Walsh, Bernd Gludovatz, and Robert O. Ritchie, "Exceptional fracture toughness of CrCoNi-based medium- and high-entropy alloys at 20 kelvin," Science, vol 378, no. 6623 (December 1, 2022), pp. 978-983, DOI: 10.1126/science.abp8070.
- Peng Zhang and Zhe-Feng Zhang, "Getting tougher in the ultracold," Science, vol 378, no. 6623 (December 1, 2022), pp. 947-948, DOI: 10.1126/science.adf2205.
- Aliyah Kovner, "Say Hello to the Toughest Material on Earth," Lawrence Berkeley Laboratory Press Release, December 8, 2022. Also here.
- Michelle Starr, "This Alloy Is The Toughest Known Material on Earth, And It Gets Tougher in The Cold," Science Alert, December 11, 2022.