
Volcanic Ash and Aviation
May 23, 2022 The most famous volcanic eruption, that of Mount Vesuvius in 79 AD, is known to most through the remains of the Ancient Roman city of Pompeii. Pompeii, now a UNESCO World Heritage Site, was buried under about five meterss (15 feet) of volcanic ash and pumice that preserved the city as it was through the two millennia to the present day. The Vesuvius eruption caused the death of Pliny the Elder (AD 23/24-79), author of the Naturalis Historia (Natural History), probably through a heart attack, while on a naval rescue mission of its inhabitants. There are many examples of how volcanic eruptions have modified human history. The island of Santorini, known in antiquity as Thera, was nearly destroyed by a violent volcanic eruption at about 1650 BC. This eruption, thought to be among the most powerful in civilized times, may have been the source of the Atlantis myth, a myth that started with an account by Plato in his Timaeus. Plato described the demise of Atlantis at about 9,600 BC, about seven millennia before his time. Atlantis sank into the ocean in a single day and night, an event consistent with a volcanic eruption. Plato also described a resultant tsunami, and evidence of this is found in the geologic record. I wrote about the Atlantis myth in an earlier article (Science and the Atlantis Myth, May 18, 2012).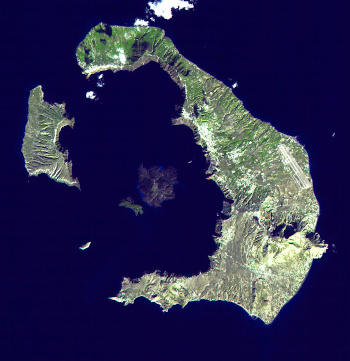
A Landsat image of Santorini (Thera), taken on November 21, 2000.
Santorini is the largest island in this image, and the smaller islands are Nea Kameni, Palea Kameni, Therasia and Aspronisi.
(NASA image via Wikimedia Commons. Click for larger image.)
There was an extreme weather event starting around the years 535-536 that's been shown to have a volcanic origin.[1-3] Europe, the Middle East, and parts of Asia were blanketed by a mysterious fog for 18 months, and tree ring studies, a traditional climate-tracking method, revealed that the years around 540 were unusually cold.[2-3] Summer temperatures in 536 were 1.5°C to 2.5°C lower, and this was the start of the coldest decade in the past 2300 years.[8-9] Ice core analysis showed that the 536 eruption was followed by two others, in 540 and 547.[8] I wrote about this volcanic influence on weather in an earlier article (The Year 536, March 18, 2019) Closer to our present time was the 1883 eruption of Krakatoa in which 70% of the Krakatoa island and its surrounding archipelago were destroyed. This volcanic eruption was heard 3,110 kilometers (1,930 miles) away in Perth, Western Australia. More than 35,000 deaths were a consequence of the eruption and the resultant tsunamis. The eruption injected large amounts of sulfur dioxide (SO2) into the stratosphere, and this was dispersed globally and converted to sulfuric acid aerosols that blocked incoming solar radiation to cause a volcanic winter. In 1884, the average Northern Hemisphere summer temperatures fell by 0.4 °C (0.72 °F).

The "afterglow" of the 1883 Krakatoa eruption, as published by the Royal Society of Great Britain Krakatoa Committee. (Illustration from "The eruption of Krakatoa, and subsequent phenomena," 1888, George James Symonds, Ed., Item 71-1250 from the Houghton Library, Harvard University, via Wikimedia Commons.)
Less than half a century ago, the continental United States experienced the Mount St. Helens eruption of May 18, 1980, an event with the thermal equivalent of 24 megatons of TNT, about 1,600 times that of the Hiroshima atomic bomb. It was reported that 57 people were killed and the economic cost in today's money was about $3.5 billion. The eruption released more than half a billion tons of volcanic ash having a composition listed in the following table. The atmospheric fine ash caused problems for internal combustion engines, other mechanical equipment, and aircraft engines.
| Oxide |
Percentage |
Melting Point (°C) |
|---|---|---|
| Silicon Dioxide (SiO2) | 65% | 1,713 |
| Aluminum Oxide (Al2O3) | 18% | 2,072 |
| Ferric Oxide (Fe2O3) | 5% | 1,539 |
| Calcium Oxide (CaO) | 4% | 2,613 |
| Sodium Oxide (Na2O) | 4% | 1,132 |
| Magnesium Oxide (MgO) | 2% | 2,852 |
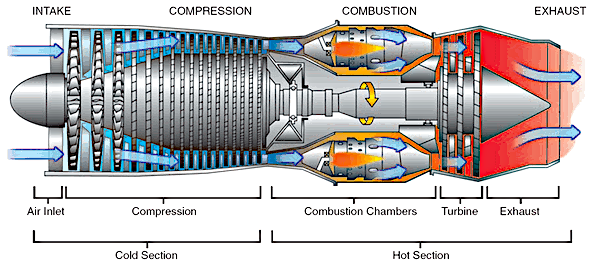
Basic components of a gas turbine engine. Federal Aviation Administration diagram, via Wikimedia Commons. Click for larger image.)
The US Navy, which is expected to fly aircraft under all conditions, has a special interest in particulate corrosion of turbine engine components by such materials as sand. Researchers from the Naval Postgraduate School (Monterey, California) and the University of California-San Diego (La Jolla, California) has recently published research on a novel idea to prevent such corrosion. The idea is that ultra-high temperature ceramics (UHTCs) might be sand-phobic; that is, molten sand won't stick to them.[4-5] I'm reminded of work I did decades ago on using single crystals of yttrium aluminum garnet (YAG, Y3Al5O12, melting point, 1940 °C) as turbine blades.[6] Sand, dust, and other particulates have been a problem for aircraft for decades. Initially, the research focus was on erosion, and engine coatings solved that problem. Now, with turbine engines operating at higher temperatures for increased power and fuel efficiency, particulates are melting when ingested into engines to cause a variety of problems.[5] filters reduce sand intake, but they're not 100% effective, and the smallest particles are the ones that pass through filters and are the quickest to melt. Research has focused on ways to quickly resolidify these through counter-reactions.[5]

A United States Marine Corps MV-22B Osprey landing in a dust cloud at Babadag, Romania.
Such clouds of potentially dangerous particulates have the potential to damage turbine engines through particulate ingestion. (US Navy image. Click for larger image.)
The research team investigated the resistance of the ultra-high temperature ceramic borides, zirconium diboride (ZrB2) and hafnium diboride (HfB2), to CMAS attack at 1000 °C, 1300 °C and 1600 °C for time up to 100 hours in air.[4] They found that these borides first oxidize before reacting with CMAS to form zirconium orthosilicate (ZrSiO4) and hafnium(IV) silicate (HfSiO4). The oxidation peaks at 1300 °C, and at 1600 °C, the reaction with CMAS to form HfSiO4 is greatly suppressed[4] ZrB2 was found to exhibit only oxidation, no reaction with CMAS, and no formation of ZrSiO4 or any other CMAS induced reaction product.[4] This finding could lead to thermal and environmental barrier coatings that prevent CMAS reaction.[4] Says Andy Nieto, Naval Postgraduate School Assistant Professor of Mechanical and Aerospace Engineering,
"We were the first to even experiment at these higher temperatures for any material for these applications... It was completely unexpected that as you would go higher in temperature, you would actually get some degree of chemical inertness from these ultra-high temperature ceramics where they were not interacting with the molten sand. It opens up a possible path forward in how we are designing these engines."[5]Their study, the first to look at the potential of utilizing ultra-high temperature ceramics in aircraft turbines, was funded by the Strategic Engineering and Research Development Program, a joint effort by the United States Department of Defense, the United States Environmental Protection Agency, and the United States Department of Energy.[5]
References:
- Ann Gibbons, "Eruption made 536 'the worst year to be alive'," Science, vol. 362, no. 6416 (November 16, 2018), pp. 733-734, DOI: 10.1126/science.362.6416.733.
- Ann Gibbons, "Why 536 was 'the worst year to be alive'," Science (November 15, 2018), doi:10.1126/science.aaw0632.
- Ultra-Precise Ice Core Sampling and the Explosive Cause of the Dark Ages – Mayewski & Kurbatov, University of Maine Climate Change News, December 18, 2018.
- Andy Nieto, Erick Samayoa. Troy Ansell, and Jian Luo, "Unusual temperature-dependent reactivity of ultra-high temperature ceramic (UHTC) borides with calcia-magnesia-alumina-silicate (CMAS)," Materialia, vol. 20 (December, 2021), Article no. 101265, https://doi.org/10.1016/j.mtla.2021.101265.
- Rebecca Hoag, "NPS Research Seeks to Advance Aircraft Turbine Resilience to Particulates," Naval Postgraduate School Press Release, March 24, 2022
- Devlin M. Gualtieri, Robert C. Morris, Dave Narasimhan, and Philip J. Whalen, "Single Crystal Oxide Turbine Blades," U.S. Pat. No. 5,573,862 (November 12, 1996).