
Colloidal Pre-Assembly
July 18, 2022 Father's Day, founded in 1910, two years after Mother's Day, was Sunday, June 19, 2022, this year. That day reminded me of some duties of fatherhood beyond bringing home the bacon (with due apology to working mothers). One of my Christmas Eve tasks was assembly of children's toys, many of which had difficult to understand assembly instructions. Assembly was facilitated by fortified eggnog and some logical reasoning.
Some assemblies are more difficult than others.
NASA's Perseverance Rover at a payload servicing facility at NASA's Kennedy Space Center on February 14, 2020.
(Wikimedia Commons image. A larger image (PIA23768) can be found on the NASA-JPL website here.)
While self-assembly of dollhouse-size objects is something that's only seen in a Jetson's cartoon, chemists and crystal growers have been performing atomic and molecular self-assembly for more than a century. One early example is the Langmuir–Blodgett film, a creation of Nobel Chemistry Laureate, Irving Langmuir (1881-1957), and physical chemist, Katharine Blodgett (1898-1979), successfully used as an antireflection coating on glass. Nanoparticles can be induced to assemble into specific shapes through the use of electric and magnetic fields. One example of this is ferrofluid, a colloidal liquid of nanoscale magnetic particles suspended in a fluid such as silicone oil.

Bluing was a common laundry item in the early to mid-20th century, but it's probably hard to find today. It added a slight blue tint to white fabrics that enhanced their appearance.
Laundry bluing is is a colloid of ferric ferrocyanide (Fe4[Fe(CN)6]3). My mother used it in the 1950s, and it was one of the chemicals in my home laboratory experiments.
Bluing can be used as a reagent for the creation of magnetic nanoparticles of ferric oxide (Fe2O3),[1] and these can be used to create a ferrofluid.
(Photograph of a bottle of Mrs. Stewart's Bluing at the Edmonds Historical Museum (Edmonds, Washington), a Wikimedia Commons image by Joe Mabel.)
A recent open access article in Science Advances by physicists, chemists and engineers at the University of Amsterdam (Amsterdam, Netherlands), the Delft University of Technology (Delft, the Netherlands), the University of Michigan (Ann Arbor, Michigan), the University of Pennsylvania (Philadelphia, Pennsylvania), and Queen's University (Kingston, Ontario, Canada) describes creation of synthetic materials from colloids of small glass particles.[2-4] As alloy designers and crystal growers know, and as phase diagrams assure us, atoms should assemble in just one way at a particular pressure and temperature condition. It is possible to cheat by adding another factor, such as stress in a thin film, to produce another phase or properties that are significantly different from a bulk material. In this way, the creation of strained silicon layers results in a semiconductor with increased electron mobility that enables faster transistor. Novel materials could be created from deposition of pre-assembled clusters, thereby bypassing the thermodynamic forces that insist that atoms and molecules are deposited in just one way. The challenge is to decouple the final material structure from its building blocks, and the approach taken in this study is preassembling clusters that are different from the bulk material and assembling these into a final material.[2] The fundamental problem that was overcome in this approach is that shape is a strong determinant of structure.[2] In their experiments, the researchers showed that they could induce colloidal shape-anisotropic building blocks to arrange into structures that are not found in bulk self-assembly.[2]
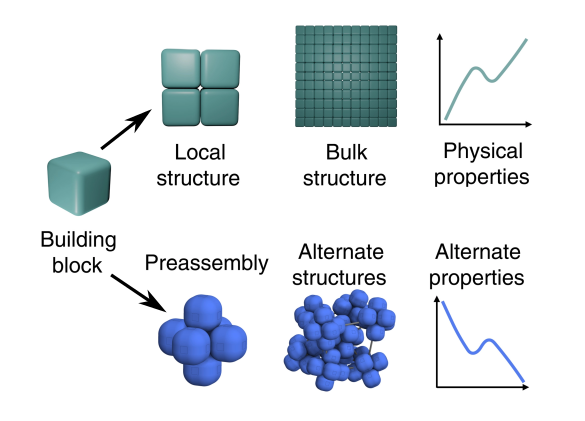
Schematic representation of the creation of a material by the usual techniques (upper steps), and from pre-assembled building blocks (lower steps). (Fig. 1 of ref. 2,[2] distributed under the Creative Commons Attribution License 4.0.)
As study co-author, Laura Rossi, an assistant professor at the Delft University of Technology, explains,
"Under certain circumstances colloids can behave like atoms and molecules, but their interactions are less strong... That makes them promising building blocks for new materials, for example for interactive materials that can adapt their properties to their environment."[4]Normally, the cube-shaped glass colloids used in this study would assemble themselves into expected simple lattice structures as dictated by their shapes.[4] However, when you assemble small groups of these colloids and combine these, they assemble into a different final structure with different material properties.[4] Says Rossi, "...We've shifted our focus to: how can we use the colloids that are already available to make interesting building blocks?"[4]
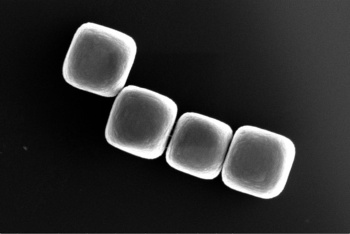
Four cubic colloids made from glass.
(TU Delft image, donated to the Public Domain.)
The research team used a technique of shape-controlled synthesis and emulsification to confine small numbers of glass colloid particles into clusters in which their arrangement is different than what's expected for bulk synthesis.[2] In experiments validated by computer simulations at Queen's University, they found that compressing colloidal balls with different degrees of sphericity in spherical confinement would generate reproducible clusters, and the structure of these are remarkably different from the bulk.[2] Computer simulations showed that clusters of six to nine particles would assemble into a material with a structure that's different from that produced by bulk assembly of similar particles.[2] One thing that they found was that the density of these structures was much lower than the density for bulk assembly,[4] and this might have application for variation of the refractive index for production of planar lenses.

scanning electron micrograph images of clusters prepared using magnetic (top row) and nonmagnetic (bottom row) particles. The configurations depend only on the number of constituent particles.
(Fig. 5b of ref. 2., distributed under the Creative Commons Attribution License 4.0. Click for larger image.)
References:
- Radek Zboril, Libor Machala, Miroslav Mashlan, and Virender Sharma, "Iron(III) Oxide Nanoparticles in the Thermally Induced Oxidative Decomposition of Prussian Blue, Fe4[Fe(CN)6]3," Crystal Growth & Design, vol. 4, no. 6 (October 5, 2004), pp. 1317-1325, https://doi.org/10.1021/cg049748+.
- Lucia Baldauf, Erin G. Teich, Peter Schall, Greg van Anders and Laura Rossi, "Shape and interaction decoupling for colloidal preassembly," Science Advances, vol. 8, no. 21 (May 27 2022), DOI: 10.1126/sciadv.abm0548. This is an open access publication with a PDF file here.
- Supplementary Materials for Ref. 2.
- New route to build materials out of tiny particles, Delft University of Technology Press Release. May 27, 2022.