
Bridgmanite
March 7, 2022 Among the artifacts of 20th century corporate research laboratories are ornate analytical balances, workaday triple beam balances, and less impressive journal routing slips. These routing slips were lists of names and associated mail drop locations that were attached to recent journal issues to allow their distribution to laboratory members. After receiving the journal issue in your mail slot, you had the obligation to quickly scan its contents, cross your name from the list, and place it in the outgoing mail. The corporate library had an arrangement in which they could make you a legal copy of any paper that caught your interest. Routing slips were another item quickly displaced by the Internet, as were many members of the library staff. I was on the routing list for New Scientist, a magazine with science and technology news, and articles of a more popular than ponderous nature. New Scientist also had some reader feedback columns, the feedback in those days done by regular mail and not email. One column that I enjoyed reading posed questions to the readership about using science to solve everyday problems. I enjoyed creating my own solutions to these questions and reading the submitted answers of those who were willing to devote enough effort to create a letter and pay for a stamp. One question was as follows. You have two geometrically equivalent spoons, one of which is sterling silver (an alloy that's 92.5% silver and 7.5% copper), and the other is the less valuable silver-plated stainless steel. How can you distinguish them? One obvious way is by weighing them, since sterling silver is about 10% more dense than stainless steel. Since most homes don't have laboratory balances, I decided on a different solution. Since the thermal conductivity of sterling silver is more than ten times that of stainless steel, you would dip them both into boiling water, and the one you would drop first would be the sterling silver spoon. As we all know, we sit on Earth's surface at the top of a very hot ball of solid and liquid materials, but it's the relatively low thermal conductivity of intermediate layers that keeps Earth's surface near our desired room temperature. The Earth's inner core is as hot as 7000 K, but it's at a pressure great enough that it exists as a solid ball of iron-nickel alloy. Just above the inner core is the outer core of liquid iron and nickel at temperatures from 3000 K - 5000 K. Above this are the upper and lower mantle of silicate minerals that comprise about two-thirds of the mass of the Earth existing from 1750 K - 3000 K. (see figure)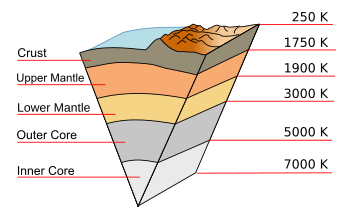
A cutaway diagram of the Earth along with temperatures along the geothermal gradient.
This diagram (except for the mountain surface features) is to scale.
(Modified Wikimedia Commons image. Click for larger image.)
The silicates that insulate us from the inner Earth are silicate perovskite ((Mg,Fe)SiO3), also known as bridgmanite, and calcium silicate perovskite (CaSiO3), also known as davemaoite, and magnesiowüstite ((Mg,Fe)O). Davemaoite is named after geophysicist, Ho-kwang (Dave) Mao (b. 1941), and bridgmanite is named after 1946 Nobel Physics Laureate, Percy Bridgman (1882-1961), who was a pioneer in high pressure research and an originator of the Bridgman-Stockbarger method of crystal growth. Bridgman was able to achieve pressures in his laboratory exceeding 10 GPa (100,000 atmospheres) that allowed the study of the physical properties of many high pressure minerals. Bridgmanite exists only at high-pressures, and it occurs at about 660 kilometers deep within the Earth where the pressure is about 24 GPa. Bridgmanite becomes unstable at higher pressures, at a depth of about 2700 kilometers. Bridgmanite constitutes around 80% of the mantle. with calcium perovskite at about 10% concentration. It is formed in the laboratory using laser heating in diamond anvil cells. Perovskite-structured (Mg,Fe)SiO3 was named bridgmanite by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association in 2014.
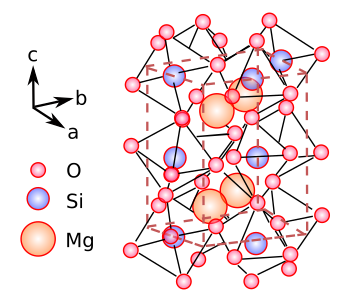
Crystal structure of bridgmanite, shown as MgSiO3.
Bridgmanite has the orthorhombic crystal structure (Space group, Pnma). It has silicon atoms surrounded by oxygen in six-fold coordination.
It's evident that only very high pressures can allow the large magnesium cations to exist in such close proximity to each other.
(Created using Inkscape from original image in ref. 1.[1] Click for larger image.)
As can be expected, the thermal conductivity of bridgmanite greatly affects the cooling rate of Earth's core. Recently, an international research team from ETH Zürich, (Zürich, Switzerland), the Carnegie Institution of Washington (Washington, DC, USA), the University of Bayreuth (Bayreuth, Germany), and Okayama University (Tottori, Japan) has used a recently developed optical absorption measurement system in a pulsed laser heated diamond anvil cell to measure the thermal conductivity of bridgmanite.[2-3] They found that the thermal conductivity of bridgmanite is about 1.5 times higher than assumed previously.[2-3] Their research is published in an open access paper in a recent issue of Earth and Planetary Science Letters.[2]
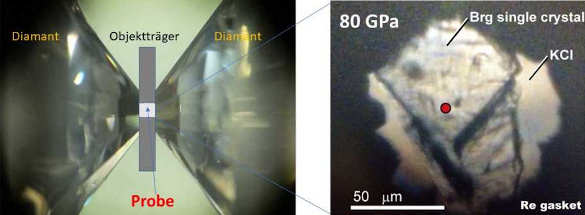
Bridgmanite in a laser-heated diamond anvil cell for thermal conductivity measurement. (ETH-Zürich image by Murakami M, et al.)
As the study authors write, the radiative thermal conductivity of bridgmanite had not been accurately measured.[2] Their measurement at 80 GPa showed that the radiative thermal conductivity of bridgmanite at the core-mantle boundary should be about 5.3±1.2 W/m-K.[2] The more rapid cooling of the mantle will result in a positive feedback process in which bridgmanite turns into the mineral, post-perovskite (another phase of MgSiO3), and the post-perovskite will accelerate cooling even further, since this mineral is a better thermal conductor than bridgmanite.[2-3] This more efficient cooling of the mantle will ultimately weaken many tectonic activities.[2] As study author and ETH professor, Motohiko Murakami, summarizes,
"Our results... suggest that Earth, like the other rocky planets, Mercury and Mars, is cooling and becoming inactive much faster than expected."[3]
References:
- What’s in a name? – Bridgmanite, Blogging a Crystal Structure a day in 2014 at the Crystallography365 website, July 8, 2014.
- Motohiko Murakami, Alexander F. Goncharov, Nobuyoshi Miyajima, Daisuke Yamazaki, and Nicholas Holtgrewe, "Radiative thermal conductivity of single-crystal bridgmanite at the core-mantle boundary with implications for thermal evolution of the Earth," Earth and Planetary Science Letters, vol. 578, Article no. 117329, January 15, 2022, https://doi.org/10.1016/j.epsl.2021.117329. This is an open access article under a Creative Commons license.
- Peter Rüegg, "Earth's interior is cooling faster than expected, ETH-Zürich Press Release, January 14, 2022.