
Tungsten
February 8, 2021 Civilization has been shaped by its materials; so much so, that some of the Ages of Man have been named by the materials used in those periods. The first such age, the Stone Age, had a duration of about three million years. Gold and silver were the first useful metals, but archaeologists don't have a Gold Age or a Silver Age in their chronology. The advent of copper metalworking gave us the Chalcolithic Age, followed by the Bronze Age.
A Stone Age mortar and pestle, c. 20,000 BC.
Stone is quite abundant, and many types of stone have mechanical properties that are sufficient for stone knives and spear heads.
This item, now at the Israel Museum (Jerusalem, Israel), stands about 29 cm high and weighs 11 kilograms (24.25 pounds).
(Wikimedia Commons image by Gary Todd
The Bronze Age was the first alloy age, since bronze is an alloy of copper with tin. Alloying with tin gives copper greater hardness and strength. Bronze exists over a range of tin concentrations, but modern bronze generally contains about 10% tin. At about the 12th century BC, the Bronze Age was followed by the Iron Age, the last Age defined by archaeologists. Iron is an abundant element, but it's hard to separate from its compounds. Its melting point, 1,538 °C (2,800 °F) is much higher than that of copper, 1,085 °C (1,985 °F). The archaeological ages persist only until the advent of written history. For that reason, the Iron Age ended during the first few centuries BC as regional histories were first written. We still use a lot of iron, today; so, it can be argued that we are still living in the Iron Age. While present society appears to be dominated by plastic, if we were to name it as a metal age, it would definitely be the Aluminum Age. Aluminum, like iron, is an abundant element, but it has advantages over iron in being lightweight, and corrosion-resistant. It was a latecomer to widespread use since, like iron, it is difficult to extract from its ores. The production of iron is by the reduction of iron oxide to pure iron, but aluminum oxide is much, much harder to reduce to produce aluminum. That's because aluminum oxide is a far more stable compound than iron oxide. At 1000 K, the free energy of formation (ΔGf) of Fe2O3 is -134.082 kcal/mole, and the free energy of formation of Al2O3 is -325.397 kcal/mole, the negative signs indicating an exothermic reaction.[1] The element, aluminum, was discovered in 1824 by Hans Christian Ørsted (1777-1851), for whom the magnetic field unit, the oersted, is named, but our Aluminum Age didn't begin until 1886 with the invention of an electrochemical process for the extraction of aluminum from molten cryolite (Na3AlF6). Application of an electric current through cryolite using a carbon cathode and anode creates liquid aluminum at the cathode. Recycling of aluminum is important, since about 25-30 kilowatt-hours of electricity are required to produce two kilograms of aluminum. This is about the daily electrical consumption of a typical US home.

Master Mechanic, P. H. McLaughlin, fitting the aluminum apex on the Washington Monument, 1884.
In 1884, aluminum was as valuable as silver (about $25/ounce in today's money), and the Washington Monument was topped with an aluminum apex in that year.
This 2.85 kg apex was about 97.5% aluminum with some alloying elements, and it was intended as a lightning rod.[2]
The electrochemical process for refining aluminum, the Hall–Héroult process, was independently invented by American chemist, Charles Martin Hall and French inventor, Paul Héroult. Hall founded the company that would become Alcoa in Pittsburgh in 1888.
(Harper's Weekly illustration by S.H. Nealy, United States Library of Congress Prints and Photographs division digital ID cph.3b44599, via Wikimedia Commons.)
Early in 2020, materials scientist, Ainissa Ramirez, who is also host of the Science Underground podcast, wrote about how another useful element, tungsten, was tamed to submission by a General Electric physicist, William D. Coolidge (1873-1975).[3] Tungsten, as most people know, is the material that made possible the production of long-lived incandescent light bulbs that initially enabled our nocturnal lifestyle. Incandescent light bulbs have been supplanted after a nearly century's long dominance by energy efficient LED lighting. Coolidge also advanced X-ray tube technology through the use of a tungsten filament and the rotating anode X-ray tube that allowed generation of X-rays of high power. He became director of the General Electric Research Laboratory in 1932, and a GE vice president from 1940-1944.
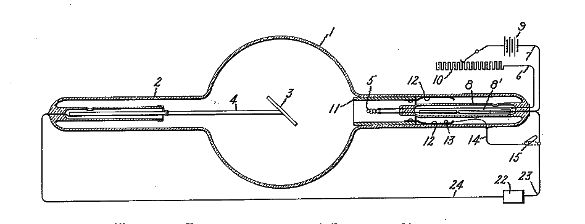
Figure 1 from US Patent No. 1,203,495, 'Vacuum Tube,' by William D. Coolidge, October 31, 1916 (Via Google Patents.[4])
The quality of light from an incandescent light bulb depends critically on the temperature of the glowing filament. A filament at 1000°C will glow orange, but white light emission requires a much higher temperature of 1300°C (see figure). The first light bulb filaments were made from carbon filaments, and the best such filaments, derived from carbonized bamboo, could function for several hundred hours. In seeking hotter, brighter, and whiter, light, manufacturers tried using high melting point metals such as osmium, tantalum, and tungsten.[3] Tungsten had all the proper properties for a filament, but it was nearly impossible to process into a fine wire.[3]
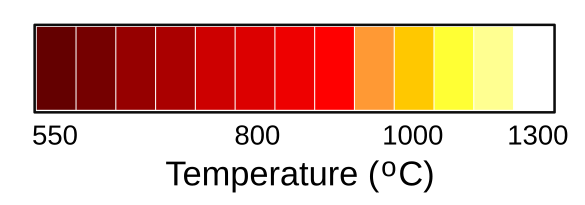
Colors associated with incandescent temperatures. The temperature scale is not linear. There will be a slight variation from material to material, since filaments are not ideal black bodies. (Modified Wikimedia Commons image by RaySys. Click for larger image.)
For those who think that student debt is just a recent problem, it was one factor in William Coolidge's decision in 1905 to accept a job at General Electric offered by his former MIT chemistry professor and first GE Research Laboratory director, Willis Whitney (1868-1958), and leave his MIT instructorship.[3] Whitney started research on light bulb filaments using the refractory metals. The term, "refractory," was apt, since these were difficult to fabricate into items such as wire. Coolidge first examined tantalum.[3] Tantalum is very easy to fashion into wire, and I've made high temperature heaters from tantalum wire. A problem that Coolidge discovered was that tantalum filaments would fail quickly when excited by alternating, rather than direct, current. Apparently, the repeated small movement caused by the magnetic field reversal in the wire from AC resulted in material fatigue that broke the filament. Coolidge then moved on to research in molybdenum and tungsten.[3] Tungsten has the highest melting point of all the elements (3,422 °C, 6,192 °F), so it can be made to glow with a white light without coming anywhere near its melting point. However, tungsten is too hard to be made into a wire using conventional wire drawing techniques. Like a good materials scientist, Coolidge tried many material processing techniques in order to make tungsten wire. He mixed tungsten powder with organic binders, but then found that he could make an amalgam of tungsten with mercury, extrude that, and then heat it to remove the mercury.[3] The tungsten filaments produced by this process worked, but they were fragile and failed under vibration.[3] Finally, the winning process was a modification of the conventional wire drawing process in which tungsten rods were made by sintering pure tungsten powder, the rods were swaged, and the heated rods were drawn down repeatedly through heated dies.[5] The resultant 6 micrometer wire became the basis of a huge market for GE, and nearly all light bulbs had tungsten filaments by 1916.[3]
References:
- L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- George J. Binczewski, "The Point of a Monument: A History of the Aluminum Cap of the Washington Monument," JOM, vol. 47, no. 11 (1995), pp. 20-25.
- Ainissa Ramirez, "Tungsten's Brilliant, Hidden History," American Scientist, vol. 108, no. 2 (March-April 2020), pp. 88-91, DOI: 10.1511/2020.108.2.88.
- William D Coolidge, "Vacuum Tube," US Patent No. 1,203,495, October 31, 1916.
- C. L. Briant, and B. P. Bewlay, "The Coolidge process for making tungsten ductile: The foundation of incandescent lighting," MRS Bulletin, vol. 20, no. 8 (August, 1995), pp. 67-73, DOI: https://doi.org/10.1557/S0883769400045164.
- Website of Ainissa Ramirez.