
Solar Cooling
November 22, 2021 After introducing the chemical elements and the periodic table, chemistry textbooks proceed to combinations of the elements to form chemical compounds. Such chemical reactions are classified as exothermic or endothermic, either releasing heat to, or absorbing heat from, the surroundings. By releasing heat, the products of the exothermic reaction decrease their enthalpy (ΔH is negative), and by absorbing heat, they increase their enthalpy (ΔH is positive). The terms, exothermic and endothermic, derive from the obvious root for temperature and the Greek prefixes, exo, (ἔξω, "external), and endo (from ἔνδον, "internal"). How can we estimate whether a reaction will be exothermic or endothermic? An easy way is to compare the sums of the atomic bond energies of the reactants and products. As a simple example, consider the reaction of hydrogen and oxygen to form water.2H2 + O2 -> 2H2OThese combinations of elements have the following bonds and bond energies.[1]
| Atomic Bond | Bond Energy (kJ/mol) |
|---|---|
| H-H | 432 |
| O-H | 467 |
| O=O | 495 |
NaHCO3 + H+ -> Na+ + CO2 + H2O

Our instant world no longer tolerates the use of a water bottle as a heating or cooling device. Instead, we have disposable chemical heating pads based on an exothermic chemical reaction, such as dissolving calcium chloride in water.
Ammonium nitrate, NH4NO3, is used in instant cold packs, since its ionic dissolution in water is an endothermic reaction that consumes 26.2 kJ/mol of heat.
I received this ammonium nitrate cold pack from a dentist after some minor oral surgery. The pack contains a pouch of water that wets the ammonium nitrate when it's broken.
(Photograph by the author.)
The endothermic reaction of ammonium nitrate with water has been proposed as a method for air cooling.[2-5] Global warming has increased the need for cooling, and much of this cooling is needed in areas with lack of access to electricity. The proposed cooling method does not require electricity, and the ammonium nitrate salt is subsequently crystallized using solar energy.[2-5] This research was published in an advanced issue of the journal, Energy & Environmental Science, by scientists from the King Abdullah University of Science and Technology (KAUST, Thuwal, Saudi Arabia) and the Hong Kong Polytechnic University (Hong Kong, China).[2] They call their process NESCOD from "no electricity and sustainable cooling on-demand.[2] One other approach to passive cooling whose principle I wrote about in an earlier article (Solar Reflecting Paint, November 19, 2018) is to radiate heat to the cold of outer space through a narrow optical passband in Earth's atmosphere at mid-infrared wavelengths (8-13 μm). However, this approach has a low intrinsic thermodynamic cooling power limit of about 160 W/m2, and it can reduce the temperature of the area beneath the cooling material by a mere 10°C.[2] The endothermic nature of ammonium nitrate mixing with water arises from the disassociation of ammonium nitrate into an ammonium cation and a nitrate anion.
NH4NO3 -> NH4+ + NO3-Ammonium nitrate has a cooling power that's greater than four times that of ammonium chloride (NH4Cl), its closest competitor.[4] The exceptional cooling power of ammonium nitrate is a consequence of its high solubility in water, which is about 2 kilograms per kilogram of water.[4-5] Another advantage of ammonium nitrate is that it's inexpensive, and it's widely used as a fertilizer.[4-5]
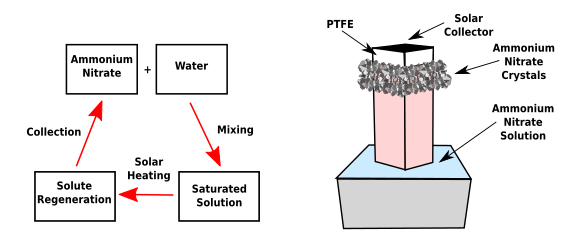
The continuous cooling device was constructed as a a tall container made of a sunlight absorbing material wrapped with a water-wicking fabric that's floated on top of the salt solution.[5] The top portion is coated with polytetrafluoroethylene (a.k.a., Teflon®) to prevent the salt crystals from climbing too high on the container.[5] (Created using Inkscape. Click for larger image.)
The continuous cooling device was constructed as a a tall container made of a sunlight absorbing material wrapped with a water-wicking fabric that's floated on top of the salt solution.[5] The salt solution wicks up the fabric, is warmed, and the water evaporates.[5] This produces ammonium nitrate salt crystals that grow on the container's outer wall.[5] It's then possible to automatically collect the crystallized salt using gravity.[4] The system's cooling power could reach up to 191 W/m2 under normal solar conditions.[2] It was found that The temperature of the cooling solution can reach about 2.4 °C in just 20 minutes.[2] This cooling system can also serve in food storage applications. When the ammonium nitrate was gradually dissolved in water in a metal cup placed inside a polystyrene foam box, the temperature of the cup dropped to around 3.6°C, and it remained below 10°C for 8 hours, and below 15°C for more than 15 hours.[4-5]
References:
- Bond Energies, Chemistry Library of the LibreTexts project.
- Wenbin Wang, Yusuf Shi, Chenlin Zhang, Renyuan Li, Mengchun Wu, Sifei Zhuo, Sara Aleida and Peng Wang, "Conversion and storage of solar energy for cooling," Energy and Environmental Science, (Advanced Online Publication, September 1, 2021. https://doi.org/10.1039/D1EE01688A. This is an open access publication with a PDF file here.
- Supplementary information for ref. 2 (PDF file).
- Strong sunlight powers passive cooling device, KAUST Press Release, September 19, 2021.
- Prachi Patel, "Salt + sunlight powers an innovative electricity-free cooling system," Anthropocene Magazine, September 23, 2021.