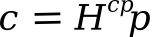Soda Fizz
September 27, 2021 Chemists have advanced our quality of life in many ways, and among the products of their research are the carbonated beverages that we enjoy on nearly a daily basis. English chemist, Joseph Priestley (1733-1804), created the first batch of carbonated water in 1767 by suspending a container of distilled water above a beer vat at a brewery. Fermentation produces carbon dioxide, which was known to Priestley as fixed air, as a product. He later improved his process by using the reaction of sulfuric acid and chalk (calcium carbonate) to produce carbon dioxide gas. Shortly thereafter, Johann Jacob Schweppe (1740-1821) used a similar process to produce carbonated mineral water. His Schweppes Company was founded in Geneva in 1783, and then relocated to London in 1792. Schweppes was designated the official drink supplier of the Great Exhibition of 1851 in London. Among the million bottles of soft drinks such as lemonade and ginger beer, sold at the Great Exhibition were many bottles of the carbonated drinks, seltzer water and soda-water. Today's global market for carbonated soft drink has an estimated value of 221.6 billion dollars.
Joseph Priestley (1733-1804).
Priestley's fame derives mostly from his discoveries of several gases, notably oxygen, which Priestley called "dephlogisticated air". The name derives from the phlogiston theory, and Priestley's belief in this theory after much evidence to the contrary caused him to become isolated from the scientific community.
Priestley's politics, which included support of the French Revolution, forced his exile from England in 1791 to Pennsylvania after a mob burned his Birmingham home.
(Priestley, commemorated in Wedgewood china, exhibited at the Chazen Museum of Art, University of Wisconsin-Madison (Madison, Wisconsin). Photo by Daderot via Wikimedia Commons
Progress in chemistry also has its history of unintended consequences, as the ozone hole and global warming have taught us. One problem with most carbonated beverages is their high sugar content, which is about 40 grams in a 12 ounce serving. This amount actually exceeds the daily added sugar allowance for an adult male recommended by many health agencies. Many people, myself included, now drink diet soft drinks, but these represent just a quarter (by volume) of the total market. Alas, I don't have a Diet Coke button on my desk, but I prefer Pepsi Zero, instead. We should be thankful for the many choices of artificial sweeteners available today. I wrote about some of these in an earlier article (Insecticidal Sweeteners, August 11, 2016). These are much better than the artificial sweeteners of my youth, which were quite awful. The older sweeteners were occasionally suspected to be toxic, at least in studies on laboratory rats. Such was the case for sodium cyclamate, which was banned in the United States in 1970 after being found to cause bladder cancer in rats at high dosage. Later studies revealed that humans did not appear to have the same problem, but cyclamates are still banned in the US. The scientific study of gases in liquids, among which were the solubility of carbon dioxide in water, followed the initial work by Priestley. Fellow English chemist, William Henry (1774-1836) formulated the eponymous Henry's law that states that the quantity of dissolved gas in a liquid is proportional to its partial pressure above the liquid;[1] viz.,
in which c is the concentration, p is the partial pressure, and the proportionality factors Hcp and Hpc are the Henry's law constants expressed as concentration change with pressure or pressure change with concentration. The term, constant, is a misnomer, since this constant is temperature-dependent. The data for the solubility of carbon dioxide in water in the following graph shows that Henry's constant Hpc is larger at higher temperature. That means that you need a greater pressure of carbon dioxide to attain a given concentration at higher temperature.
or
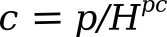
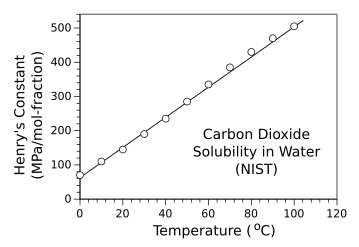
Henry's Law constant Hpc for carbon dioxide in water as a function of temperature.
(Data from a study by the US National Bureau of Standards and Technology (Ref. 2), plotted using Gnumeric. Click for larger image.)
Returning to the object of our desire, a refreshing drink of carbonated beverage, we note that there are about 7.5 grams of carbon dioxide (0.170 moles) per liter in an especially bubbly mix. Using the data from the graph above, the Henry's law constant at room temperature is about 135 MPa/mole-fraction. Then, by calculating the moles of water in a liter and applying the Henry's law equation, we get a pressure of 0.413 MPa (4.075 atmospheres, or 59.891 psi). We can easily see why gas escapes when we first open a bottle of soda, since the internal pressure is quite a bit larger than atmospheric pressure. It's noted that PET (polyethylene terephthalate) plastic soda bottles will withstand about 150 psi internal pressure; so, we're well within safety limits for bottles around room temperature. The graph above shows that for a rough estimate, a temperature of about 60 °C leads to bottle failure, the PET material properties assumed to be constant. One question that I and many soda enthusiasts have is why soda goes flat in a tightly sealed bottle. It's an annoyance when you find that half of the contents of your carefully stored two liter soda bottle is too flat to drink. The problem is the dead space in the bottle, which is replenished by gas from the liquid. I did a computer simulation of how much of the initial carbon dioxide remains in the liquid in a two liter bottle as a function of the number of times it's opened (source code here). While the simulation is based on some simplification of the process; namely, that no gas escapes from the liquid as a serving is poured, and that the gas and liquid attain equilibrium between servings. However, the results, as shown in the graph, confirm our expectation that after five one-cup servings, when half the bottle contents still remains, our soda is flat. The recommendation is that unless you're filling many cups after the first opening, you should always buy single-serve bottles if you enjoy maximum fizz.
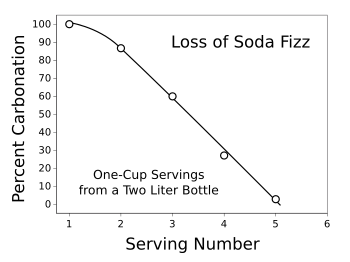
percent of initial carbonation remaining after repeated opening of of a two liter soda-water bottle.
At the fifth serving, when half the contents are still in the bottle, there is essentially no ccarbon dioxide in the soda.
(Graphed using Gnumeric from data given by the computer simulation.)
References:
- W. Henry, "Experiments on the quantity of gases absorbed by water, at different temperatures, and under different pressures," Phil. Trans. R. Soc. Lond., vol. 93 (January 1, 1803), pp, 29-43, https://doi.org/10.1098/rstl.1803.0004.
- John J. Carroll, John D. Slupsky, and Alan E. Mather , "The Solubility of Carbon Dioxide in Water at Low Pressure," Journal of Physical and Chemical Reference Data, vol. 20, no. 8 (1991), pp. 1201-1209, https://doi.org/10.1063/1.555900 (PDF file at NIST).
- Andrea Becker, "How Much Pressure Can a Two Liter Bottle Handle?" at seattlepi.com.