
Elastocaloric Effect
January 6, 2020 Household refrigerators, and most other refrigerators, use the expansion of a gas such as freon (1,1,1,2-Tetrafluoroethane, R-134a) to cause cooling by the well-known thermodynamic process of free expansion. Freon R-134a has the low boiling point of −26.3 °C and a latent heat of vaporization at its boiling Point of 51.9 kcal/kg. This is nearly as large as the heat of fusion of water at its freezing point, 79.7 kcal/kg. In the 1990s, when I was doing research on the magnetic and hydrogen storage properties of alloys of the rare earth elements with other metals, some of my colleagues were doing research on using these materials for a different type of refrigeration, magnetic refrigeration. Magnetic refrigeration is based on the magnetocaloric effect which was discovered in 1881 by German physicist, Emil Warburg. Warburg found that iron subjected to an applied magnetic field of 10,000 gauss would cool about a degree Celsius. For iron, the effect is small, considering that the Earth's magnetic field is about half a gauss. The thermodynamics of the refrigeration cycle are shown in the figure, and it's essentially a way of using the entropy associated with the alignment of the magnetic moments of the atoms in a solid as a heat pump.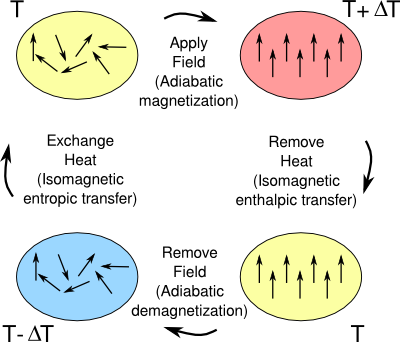
The thermodynamic cycle of a magnetic refrigerator.
The magnetocaloric effect uses entropy as a means of extracting heat from a substance. The applied magnetic field aligns the magnetic moments of the atoms in a solid, and this results in a temperature change.
Magnetic refrigeration was first used as a means of cooling small volumes to temperatures near absolute zero, a method developed by chemist and Nobel laureate, William Giauque (1895-1982).
(Click for larger image.)
The efficiency of a magnetic refrigerator is dependent on the magnetic material, and there are many materials that demonstrate a better effect than iron. The magnetocaloric effect is greatest near the vicinity of a magnetic phase transition, such as the Curie temperature at which the ferromagnetic order is lost and entropy becomes large. Some alloys of gadolinium have a Curie temperature near room temperature, and they exhibit a "giant magnetocaloric effect." Here's a table of properties of some of these materials, as taken from a previous article (Magnetocaloric & Electrocaloric Effects, April 25, 2011).[2] In this table, TC is the Curie temperature in kelvin, and ΔSM is the transition entropy in Joule-kg-1K-1.
| Magnetic Material | TC(K) | ΔSM(Jkg-1K-1) |
|---|---|---|
| Gd | 294 | 10.2 |
| Gd0.5Dy0.5 | 230 | 10.2 |
| Gd0.74Tb0.26 | 280 | 11.5 |
| Gd7Pd3 | 323 | |
| Gd5(SixGe1-x)4 at x=0.43 | 247 | 39.0 |
| Gd5(Si1.985Ge1.985Ga0.03)2 | 290 | |
| Ni52.6Mn23.1Ga24.5 | 300 | 18.0 |
| MnAs0.9Sb0.1 | 286 | 30 |
| MnFeP0.45As0.35 | 300 | 18.0 |
| La1-xCaxMnO3 at x=0.33 | 267 | 6.4 |
| La0.9K0.1Mn0.3 | 283 | 1.5 |
| La0.75Ca0.15Sr0.1Mn0.3 | 327 | 2.8 |
| La0.677(Ca,Pb)0.333Mn0.3 | 296 | 7.5 |
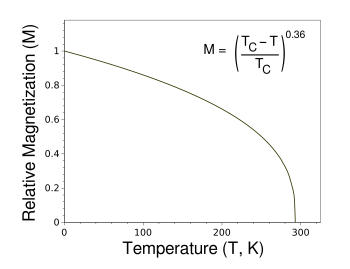
Ideal magnetization curve of gadolinium as a function of temperature.
The function shown gives the temperature dependence of magnetization of a ferromagnetic material according the the mean-field approximation.
(Created using Gnumeric.)
There's another effect that's the electric analog of the magnetocaloric effect. In the electrocaloric effect, materials show a reversible temperature change in response to an applied electric field, just as magnetic materials have a reversible temperature change in response to a magnetic field. There's a change of the system entropy as the electric dipoles in the material align themselves with the applied field. The piezoelectric material, lead zirconate titanate (PZT) demonstrates a cooling of more than 12 °C when a field of 480 kilovolts per centimeter is applied at 215 °C.[2] Barium titanate (BaTiO3) is another electrocaloric material.[3] There are other "caloric" effects besides magnetocaloric and electrocaloric, one of which is the elastocaloric effect. Like the other "caloric" effects, a rapid change of an external field alters the system entropy, and for elastocalorics it's the stress field that changes, with its effect manifest in the material strain.[5] In 2015, a research team from the Technical University of Denmark (Roskilde, Denmark) examined the elastocaloric effect in wires of the superelastic alloy NiTi, which is also known as a shape-memory alloy.[4-5] Motivations for the study were the greater amount of latent heat released during the elastocaloric effect than the magnetocaloric effect and its potentially higher power density.[5] At a cycle rate of 2 Hz, NiTi elastocaloric devices in wire form had a specific cooling power that was 70 times better than gadolinium magnetocaloric devices. The specific cooling power was 7 kWh/kg, as compared with 0.1 kWh/kg for gadolinium. The elastocaloric devices, however, have a short fatigue life, and they need large tensile forces to operate.[5] The NiTi devices function by a martensitic transformation, a reversible solid-state phase transformation between two different crystal phases that happens when a mechanical stress of about 15,000 psi (about 100 MPa) is applied. This martensitic transformation is also responsible for the temperature-induced shape memory effect and stress-induced superelasticity.[5] When stress causes the material in its austenitic phase to transform to martensite, it heats. If the heat is extracted, and the stress removed, there's cooling during the reverse transformation.[5] The NiTi wires were first subjected to 400 training cycles in which they were stressed (loaded), and then unstressed (unloaded), at various temperatures to stabilize their superelastic behavior.[4] The largest measured adiabatic temperature change during loading was 25 °C, with a corresponding 21 °C change during unloading (at 48.85 °C). Experiments showed that there are two sources of temperature irreversibility; namely, the hysteresis in the stress-strain curve, and a temporary residual strain left after unloading. The residual strain produces a temporary bending of the wire and a reduced temperature change.[4] The Danish researchers propose guidelines about the required material properties for an efficient elastocaloric cooling device.[4] They point out the this refrigeration technology can be used in space applications, since it's independent of gravity and could be highly reliable.[5] The next step is to address the problem of limited fatigue resistance.[5] While the NiTi devices depend on a phase transformation in their elastocaloric effect, a recently published article in Science utilizes just an entropy change in the same effect.[6-8] The research, on something as simple as a twisting a rubber band (see figure), was performed by a huge team of scientists from Nankai University (Tianjin, China), the University of Texas at Dallas (Richardson, Texas), Wuhan University (Wuhan, China), the Tsinghua Shenzhen International Graduate School (Shenzhen, China), Tsinghua University (Beijing, China), the State University of Campinas (Campinas, Brazil), Georgia Southern University (Statesboro, Georgia), MilliporeSigma (Milwaukee, Wisconsin), Tianjin University of Technology (Tianjin, China), Lintec of America (Richardson, Texas), the University of Science and Technology Liaoning (Anshan, China), and the China Pharmaceutical University (Nanjing, China).
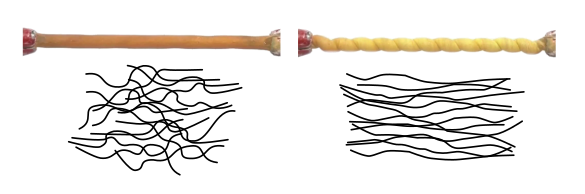
Twisted rubber band with an illustration of how stretch affects the molecular chains and system entropy. (Created using Inkscape and portions of screenshots from a University of Texas at Dallas video.)
As a result of the entropy change, stretched rubber bands will extract heat from their surroundings as they are allowed to relax.[6] The research team's experiments went beyond simple stretching to twisted, coiled, and supercoiled fibers of natural rubber, nickel titanium wires, and nylon and polyethylene fishing line.[6-7] Supercoiling is when the twisted fibers coil around themselves and coil around the coils.[7,9] For each material, 3-centimeter lengths were pulled taut, and then wound with a rotary tool.[7] The different fibers warmed up by as much as 15°C. When allowed to unwind, the fibers cooled by the same amount.[7] To properly assess their cooling potential, fibers were twisted and untwisted in the water bath of a calorimeter, and the rubber fibers produced about 20 joules of heat energy per gram, which was about eight times more energy than the rotary tool expended in their twisting.[7] The other materials had similar performance, which was comparable to the efficiency of standard refrigerants.[7] To examine the molecular basis of the effect, the research team used X-ray analysis of the molecular structure of the material fibers.[7] An elastocaloric system based on twist is more compact than one based on stretch.[7] Rubber needs to be stretched to seven times its length to get the same results.[7] A small refrigerator, about the size of a ballpoint pen cartridge, created with twisted nickel titanium wires, was built, and this cooled a small volume of water by 8°C in a few seconds.[7]
References:
- Engin Gedik, Muhammet Kayfeci, Ali Kecebas and Hüseyin Kurt, "Magnetic Refrigeration Technology Applications On Near-room Temperature," Fifth International Advanced Technologies Symposium (IATS'09), May 13-15, 2009, Karabuk, Turkey. (This reference appears to be no longer available online.)
- A. Mischenko, Q. Zhang, J.F. Scott, R.W. Whatmore, and N.D. Mathur, "Giant Electrocaloric Effect in Thin-Film PbZr0.95Ti0.05O3," Science, vol.311, no. 5765 (March 3, 2006) pp. 1270-1271. Also appears as A. Mischenko, Q. Zhang, J.F. Scott, R.W. Whatmore, and N.D. Mathur, "Giant electrocaloric effect in thin film Pb Zr_0.95 Ti_0.05 O_3," arXiv, November 19, 2005.
- Lluís Mañosa, Antoni Planes, and Mehmet Acet, "Advanced materials for solid-state refrigeration," J. Mater. Chem. A, 2013, 1, pp. 4925-4936, DOI: 10.1039/C3TA01289A; Also appears as Lluis Manosa, Antoni Planes, and Mehmet Acet, "Advanced materials for solid-state refrigeration," arXiv, March 15, 2013.
- J. Tušek, K. Engelbrecht, L. P. Mikkelsen and N. Pryds, "Elastocaloric effect of Ni-Ti wire for application in a cooling device," J. Appl. Phys., vol. 117 (2015), article no. 124901, http://dx.doi.org/10.1063/1.4913878.
- Squeeze to remove heat: Elastocaloric materials enable more efficient, 'green' cooling, American Institute of Physics Press Release, March 25. 2015. Public Release: 24-Mar-2015
- Run Wang, Shaoli Fang, Yicheng Xiao, Enlai Gao, Nan Jiang, Yaowang Li, Linlin Mou, Yanan Shen, Wubin Zhao, Sitong Li, Alexandre F. Fonseca, Douglas S. Galvão, Mengmeng Chen, Wenqian He, Kaiqing Yu, Hongbing Lu, Xuemin Wang, Dong Qian, Ali E. Aliev, Na Li, Carter S. Haines, Zhongsheng Liu, Jiuke Mu, Zhong Wang, Shougen Yin, Márcio D. Lima, Baigang An, Xiang Zhou, Zunfeng Liu, and Ray H. Baughman, "Torsional refrigeration by twisted, coiled, and supercoiled fibers," Science, vol. 366, no. 6462 (October 11, 2019), pp. 216-221, DOI: 10.1126/science.aax6182.
- George Musser, "A fridge made from a rubber band? Twisted elastic fibers could cool your food," Science, October 10, 2019, doi:10.1126/science.aaz8133.
- “Twistocaloric” effect could usher in new wave of cooling technology free of greenhouse gases, YouTube Video by the University of Texas at Dallas, October 10, 2019.
- A colleague of mine who had an interest in model aircraft published an article on the twisting of rubber bands. Robert Morris, "Twist and Writhe near Max Turns in a Rubber Motor," Free Flight Quarterly, vol. 39 (April 2011), pp. 20-22. Part 2 of the article is in vol. 52 (July, 2014).