
Ice XVIII
June 24, 2019 The past few weeks of warm weather at Tikalon's home in the Northeastern United States makes it seem that winter was just a bad dream. We can expect much lower home heating bills; and, also, safer automobile driving on roads devoid of ice and snow. While winter travel hazards of snow and heavy ice on road surfaces are easy to see, we're sometimes surprised by black ice on apparently clear roadways. Black ice is the easily unnoticed thin layer of ice that sometimes forms after a light rain on road surfaces that are below the freezing temperature of water.
A common road sign in the Northeastern United States.
Black ice will form on any road surface during light rain when the temperature of the road surface is below the freezing temperature of water, bridge surfaces will often cool to such temperatures before the rest of the road.
That's because frigid air cools the bridge from both above and below.
(Wikimedia Commons image.)
What is called black ice is not a unique phase of ice. It's just the ubiquitous hexagonal crystalline form of solid water, Ice Ih, that we encounter in our normal environment. Because of its simple molecular structure and hydrogen bonding, water is a strange material, and it's most unusual properties are noted on many websites. Its most unusual property is that its solid form is less dense that the liquid, so ice floats. This property was very important to the evolution of life on Earth. The ice that we know is just one of many of the solid forms of ice; but, since these exist at temperatures and pressures far beyond room temperature and atmospheric pressure, they are found only in the laboratory. The temperature and pressure conditions for the existence of these many forms of ice can be found in the phase diagram of water shown below,
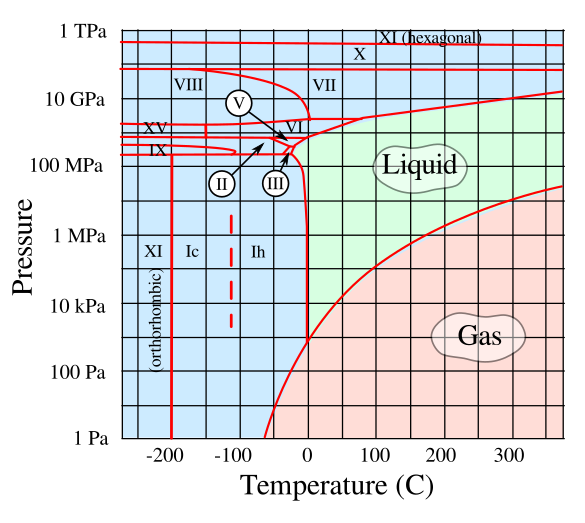
Phase diagram of water up to a pressure of 1 TPa (10 Mbar). The solid forms of water, not all of which are shown here, are Ice Ih, the ubiquitous hexagonal crystalline form of ice; ice Ic, a form of ice in which the oxygen atoms are arranged in the diamond cubic structure; ice II, a rhombohedral crystalline form of ice; ice III, a tetragonal crystalline ice that's denser than water; ice IV, a metastable rhombohedral phase of ice; ice V, a monoclinic crystalline phase of ice; ice VI, a tetragonal crystalline phase of ice; ice VII, a cubic phase of ice; ice VIII, a version of ice VII with a more orderly atomic arrangement; ice IX, a tetragonal phase of ice; ice X, a phase of ice that forms at about 70 GPa; ice XI, an orthorhombic crystalline form of ice that's the most stable configuration of ice Ih; ice XII, a tetragonal, metastable, dense crystalline phase of ice that's about 1.3 times denser than water; ice XIII, a monoclinic crystalline phase of ice; ice XIV, an orthorhombic crystalline phase of ice; Ice XV, a phase of ice VI in which the hydrogen nuclei are well ordered; and ice XVI, the ice having the least density. (Rendered using Inkscape from data at Wikimedia Commons by Cmglee. Click for larger image.)
As can be seen from the above phase diagram, there's a solid form of water, ice IX, that's stable at very low temperatures at pressures between 200 and 400 MPa. This tetragonal crystal has a density of 1.16 g/cc, which is 26% higher than ordinary ice. While few people are familiar with ice IX, many people are familiar with the fictional material, ice-nine, from Kurt Vonnegut's novel, Cat's Cradle. As I wrote in an earlier article (Fictional Materials, April 21, 2016), Vonnegut's phase of ice has the dangerous property that it will spontaneously crystallize water into ice-nine under normal temperatures and pressures, but it will only melt at 45.8 degrees Celsius, slightly above Earth's ambient temperature. As you can see, this would cause serious problems for Earth's ecosystem. Vonnegut said that the ice-nine idea came from Nobel Chemistry Laureate, Irving Langmuir of General Electric's research laboratory. Vonnegut was familiar with Langmuir's work, since he had worked in the public relations office at GE in Schenectady, New York. As the story goes, Langmuir suggested the idea of an ice that's solid at room temperature to H.G. Wells when he visited the laboratory. Since Langmuir and Wells never published anything about ice-nine, Vonnegut decided to use the idea himself after the deaths of both Langmuir and Wells. The character of the ice-nine inventor, Dr. Felix Hoenikker, is based on Langmuir.
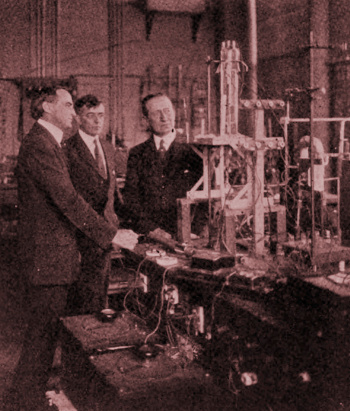
Left to right, Willis R. Whitney, Director of the General Electric research laboratory, Irving Langmuir, and Guglielmo Marconi, August, 1922.
They're looking at a recently developed 20 kilowatt triode vacuum tube.
Both Langmuir and Marconi were Nobel Laureates, Langmuir in chemistry and Marconi in physics.
(Wikimedia Commons image, modified for artistic effect.)
One unique solid phase of water, ice XVIII, also known as superionic water, exists at extremely high temperatures and pressures. Ice XVIII consists of oxygen ions in a crystal lattice with the hydrogen ions floating freely within the lattice interstices. This free movement of hydrogen ions is analogous to the movement of electrons in a metal, so superionic water is extremely conductive. Ice XVIII was conjectured to exist for many years before high pressure laboratory techniques had advanced to the stage at which it could be experimentally demonstrated. Interestingly, since it's a conductive material, ice XVIII would actually be black in color, just as conductive ceramics are black. Now a team of scientists and engineers from Lawrence Livermore National Laboratory (Livermore, California) and the University of Rochester (Rochester, New York) have successfully created ice XVIII and verified its creation using in situ X-ray diffraction.[1-3], many years after its theoretical prediction in 1988.[4] They used high-powered lasers to shock wave compress water to 100-400 GPa and 2,000-3,000 K, creating a nanosecond instance of this water phase.[1-2] Says LLNL physicist, Federica Coppari, a lead author of the paper, "...compressing water to such pressures and temperatures and simultaneously taking snapshots of the atomic structure was an extremely difficult task, which required an innovative experimental design."[2] The possibility of the existence of ice XVIII started with the 1988 computer simulations of Pierfranco Demontis, who was then at the University of Pennsylvania (Philadelphia, Pennsylvania)).[4] These simulations showed the possibility of superionic ice with a conductivity above 100 siemens/centimeter, which is almost as high as that of a metal.[1] The current experiment builds on one conducted last year for which indirect evidence for ice XVIII was obtained.[5] In that experiment, water was first compressed mechanically to about 2 GPa, using a diamond anvil cell. The cell, containing ice VII, was then blasted with a laser to create a conductive ice.[3] The current experiment skipped the mechanical squeeze and laser blasted water contained in a diamond anvil cell. Additional laser beams were used to vaporize a nearby iron foil, and this process generated X-rays for analysis of the water specimen compressed to 100-400 GPa and heated to 3,000-5,000 degrees Fahrenheit.[2,3] The X-ray diffraction pattern confirmed the new face-centered cubic phase of ice.[1] Says Coppari,
"The x-ray diffraction patterns we measured are an unambiguous signature for dense ice crystals forming during the ultrafast shockwave compression demonstrating that nucleation of solid ice from liquid water is fast enough to be observed in the nanosecond timescale of the experiment."[2]
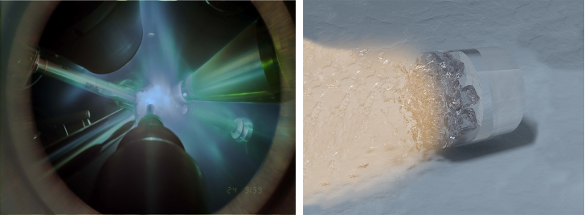
Creation of ice XVIII. The left image is a photograph of the X-ray diffraction experiment in which high power lasers are focused on the water sample to compress it into the superionic ice XVIII phase. Additional lasers irradiate an iron foil to create the X-rays for analysis. The right image is an artist's impression of the laser compression experiment in which water in a diamond anvil cell is compressed by shock waves and heated to produce the conditions for ice XVIII formation. (Left image created by Marius Millot/Federica Coppari(LLNL) and Eugene Kowaluk (LLE); right image created by Marius Millot, Federica Coppari, Sebastien Hamel, and Liam Krauss. Click for larger image.)
Ice XVIII might be a common phase of water in the universe, since it might exist within large planets where pressures and temperatures are within the range for its formation, examples being our Solar System's own Uranus and Neptune.[3] Measurements of the magnetic fields of these planets have shown complex and uneven structures that might be explained by an interior layer of superionic ice.[2-3,7] Ice XVIII is just another phase of water, but it behaves as if it were a new state of matter.[3]
References:
- Marius Millot, Federica Coppari, J. Ryan Rygg, Antonio Correa Barrios, Sebastien Hamel, Damian C. Swift, and Jon H. Eggert, "Nanosecond X-ray diffraction of shock-compressed superionic water ice," Nature, vol. 569, no. 7755 (May 8, 2019), pp. 251-255, https://doi.org/10.1038/s41586-019-1114-6.
- Research Reveals Atomic Structure of Superionic Ice, Lawrence Livermore National Laboratory Press Release, May 8, 2019.
- Joshua Sokol, "Black, Hot Ice May Be Nature's Most Common Form of Water," Quanta Magazine, May 8, 2019.
- Pierfranco Demontis, Richard LeSar, and Michael L. Klein, "New High-Pressure Phases of Ice," Physical Review Letters, vol. 60, no. 22 (May 30, 1988) Article no. 2284, https://doi.org/10.1103/PhysRevLett.60.2284.
- Superionic Ice Computer Simulation, YouTube Video by Millot, Coppari, and Kowaluk (LLNL), May 8, 2019.
- Breanna Bishop, "First experimental evidence for superionic ice, Lawrence Livermore National Laboratory Press Release, February 5, 2018.
- Li Zeng and Dimitar Sasselov, "The effect of temperature evolution on the interior structure of H2O-rich planets," The Astrophysical Journal, vol. 784, no. 2 (March 10, 2014); also at arXiv.