
Boron Nitride Aerogels
April 1, 2019 While many people scan the ingredient list of the foods that they buy, looking for such things as allergens, excess fat, and excess sugar, there's one "ingredient" that's often overlooked - air. While the carbon dioxide in carbonated beverages is listed as the first ingredient in the form of carbonated water, the carbon dioxide byproduct of yeast fermentation that adds volume to bread is not listed, just the yeast. Carbon dioxide is also used to replace air in food packaging to increase shelf life, in which case the gas must meet a purity standard.
One food allergic reaction common to about 1% of the population is gluten intolerance.
Gluten is a protein found in the starch of cereal grains such as wheat, barley, rye, and related species.
A common food ingredient, modified food starch, may also be derived from these grains, but some manufacturers use modified corn starch instead. (USDA image.)
Home chefs have encountered the delight of air as an ingredient in whipped cream and soufflés that incorporate air by mechanical aeration; a.k.a., whisking. The soufflé originated in 18th century France, and its name derives from the French word for "inflate." In my childhood, in the days of simple visual comedy, the accidental deflation of a soufflé by a loud noise was often invoked, usually during a chef's or diner's appreciative gaze. Just as the addition of air to food can give it some desirable properties, the same is true for inorganic materials. Aerogels are ultralight porous synthetic materials created by replacement of the liquid in a gel by gas using the process of supercritical drying. Aerogels, which appear optically to be frozen smoke, have a low density as a consequence of their being more than 99% gas. This low density, coupled with the low thermal conductance of the entrapped gas, gives aerogels a low thermal conductivity. Aerogels resist mechanical force at low loading, have a plastic yield at intermediate loading, and show catastrophic failure at high loading. The reduced thermal conductivity of aerogels derives from an interesting effect. Knudsen flow, named after Martin Knudsen (1871-1949), who is the inventor of the Knudsen cell used as a vapor source in molecular-beam epitaxy, describes a fluid flow state in which the molecular mean free path length is of the same order as the physical length of its constraints. In aerogels, the gas is trapped in pores about 100 nanometers in size, so the gas molecules are ineffective transporters of heat. The thermal conductivity of air is reduced by a significant factor when the air is constrained to a 10-100 nanometer pore.[1] Silica aerogels have an optical transmittance of about 99% and a refractive index of only 1.05 (pure silica has a refractive index of about 1.55). The nanoscale pore size, which is smaller than a wavelength of visible light, is responsible for the general transparency of aerogels. Aerogels typically have a pale blue color that arises from Rayleigh scattering of shorter wavelengths by the nanoscale pores. Rayleigh scattering is what causes the blue color of the daytime sky. As I wrote in an earlier article (Stardust, August 20, 2014), aerogels were used to capture interplanetary dust in the NASA Stardust spacecraft. Since cosmic dust grains travel at speeds of up to 5 kilometers/second, the aerogel was designed to gently slow the cosmic dust grains for capture. The aerogel detector, a portion of which is shown in the photograph, was made from ninety blocks of silica aerogel having 99.8% empty space and just 0.01% the density of silica.
Aerogel cosmic dust capture medium in the NASA Stardust spacecraft.
Aerogel was also used as thermal insulation for the Mars Rovers.
(NASA Image)
As the Stardust cosmic dust capture device demonstrates, there are quite a few applications other than thermal insulation for a functional material that's mostly empty space. An aerogel is used as a Cherenkov radiation detector in the Belle Experiment. One side effect of the pore structure of aerogels is their high surface area. As I wrote in a previous article (Carbon Nano-Aerogel, March 14, 2011), carbon aerogels with high surface area allow creation of high performance electrodes for electric double-layer capacitors (supercapacitors). While silica aerogels are inexpensive and easy to manufacture, aerogels are made from other inorganic materials, such as alumina, chromia, tin dioxide, and the aforementioned carbon. It's possible to make aerogels of boron nitride, also; and, in 2015, an international team of scientists from the United States and Australia created a boron nitride aerogel that functions as a sponge for oil.[2-5] This aerogel has a density that's 1,500 times less than the density of bulk boron nitride and a pore structure that allows absorption of more than 33 times their weight in organic solvents, such as oil. A mechano-chemical exfoliation and a chemical functionalization are required to make this material water-dispersible.[2,4] Aerogels would be more useful as thermal insulators if their thermal and mechanical properties were improved. A large international team of scientists has developed an improved boron nitride aerogel having a negative Poisson's ratio, which dramatically improves its mechanical stability.[6-8] The research team has members from the University of California (Los Angeles, California), the Harbin Institute of Technology (Harbin, P. R. China), Lanzhou University (Lanzhou, P. R. China), the University of California (Berkeley, California), Southeast University (Nanjing, P. R. China), Hunan University (Changsha, P. R. China), the New Jersey Institute of Technology (Newark, New Jersey), Purdue University (West Lafayette, Indiana), King Saud University (Riyadh, Kingdom of Saudi Arabia), and Lawrence Berkeley National Laboratory (Berkeley, California).[6] Materials with a negative Poisson's ratio are called auxetic materials. Poisson's ratio, which is usually symbolized by the symbol, ν, is the measure of how much the material will shrink in a plane when you pull on it. Technically, it's the ratio of the contraction in the x- or y-direction to the extension in the z-direction, but there's a negative sign thrown in so that the ratio is positive for normal materials. Our usual experience is that materials get thinner as we pull on them, and Poisson's ratio is usually in the range of about 0.3 for most metals. The negative Poisson's ratio auxetic materials have the counter-intuitive property of fattening when they're pulled. I discussed auxetic materials in an earlier article (Auxetics, May 13, 2011).
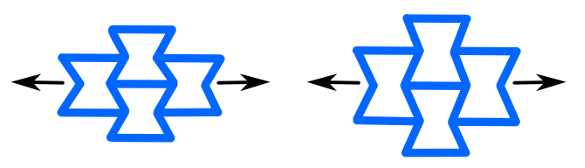
Two-dimensional model of an auxetic materials. Pulling it from the sides causes an expansion in the vertical direction, which is opposite to a "normal" material's response. (Created using Inkscape. There's a YouTube demonstration at Ref. 9.[9])
Auxetic materials have enhanced impact resistance. Unlike normal materials that spread away from an indenter, auxetic materials move towards the indent and act to block the indenter. The boron nitride aerogels were produced with a Poisson's ratio of −0.25.[6] This gives the material more flexibility than other ceramic aerogels, and it makes it less brittle.[8] The boron nitride aerogel can be compressed to 5% of its original volume with full recovery after the compression is removed, while other aerogels can recover after only about 20% compression.[8] This novel aerogel was created by using a three-dimensional graphene structure as a template to create nanolayered double-pane walls of atomically thin sheets of hexagonal boron nitride with hyperbolic surfaces.[6] A hyperbolic surface is a saddle shape with a negative curvature.[7] This hyperbolic structure gave the material mechanical and thermal resistance properties, including elasticity and resistance to repeated temperature spikes, that exceed those of current aerogels.[7-8] The material has a negative thermal expansion coefficient; that is, it contracts rather than expands as other ceramics do with an increase in temperature.[8] The thermal expansion coefficient was −1.8 x 10-6 per degree-C.[6]
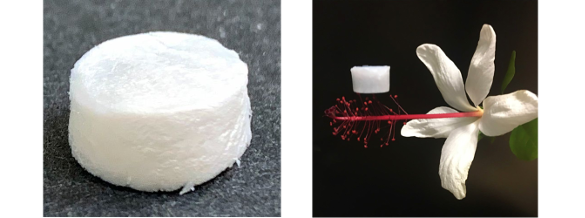
Pellets of the boron nitride aerogel. Since the material is 99% air by volume, it can sit atop a delicate flower without damaging it. (Left image by Oszie Tarula and right image by Xiangfeng Duan and Xiang Xu, both via UCLA.)
The boron nitride aerogel has a density of about 0.1 milligrams per cubic centimeter, it's elastic up to 95%, resists rapid thermal shocks of 275°C per second and survives 1400°C.[6] Its thermal conductivity is about 2.4 milliwatts per meter-kelvin (mW/m-K) in vacuum, and about 20 mW/m-K in air.[6] The material loses less than 1 percent of its mechanical strength after a week-long exposure to 1,400°C.[8] Such a material would be useful as a thermal insulator for spacecraft.[6] As study author, Xiangfeng Duan, of the University of California, Los Angeles, says,
"The key to the durability of our new ceramic aerogel is its unique architecture... Its innate flexibility helps it take the pounding from extreme heat and temperature shocks that would cause other ceramic aerogels to fail... Those materials could be useful for thermal insulation in spacecraft, automobiles or other specialized equipment... They could also be useful for thermal energy storage, catalysis or filtration."[8]This research was funded by the National Science Foundation, among other sources.
References:
- Axel Berge and Pär Johansson, "Literature Review of High Performance Thermal Insulation," Chalmers University of Technology (Gothenburg, Sweden), Report 2012:2 (PDF File).
- Weiwei Lei, Vadym N. Mochalin, Dan Liu, Si Qin, Yury Gogotsi, and Ying Chen, "Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization," Nature Communications, vol. 6, article no. 8849 (November 27, 2015), doi:10.1038/ncomms9849. This is an open access publication with a PDF file available here.
- Supplementary figures for ref. 6.
- Drexel Materials Scientists Aid Australian Institution in Developing Super-Absorbent Material That Can Soak Up Oil Spills, Drexel University Press Release, November 30, 2015.
- Deakin scientists create revolutionary material to clean oil spills, Deakin University Press Release, November 30, 2015.
- Xiang Xu, Qiangqiang Zhang, Menglong Hao, Yuan Hu, Zhaoyang Lin, Lele Peng, Tao Wang, Xuexin Ren, Chen Wang, Zipeng Zhao, Chengzhang Wan, Huilong Fei, Lei Wang, Jian Zhu, Hongtao Sun, Wenli Chen, Tao Du, Biwei Deng, Gary J. Cheng, Imran Shakir1, Chris Dames, Timothy S. Fisher, Xiang Zhang, Hui Li, Yu Huang, and Xiangfeng Duan, "Double-negative-index ceramic aerogels for thermal superinsulation," Science, vol. 363, no. 6428 (February 15, 2019), pp. 723-727, DOI: 10.1126/science.aav7304
- Manish Chhowalla and Deep Jariwala, "Hyperbolic 3D architectures with 2D ceramics," Science, vol. 363, no. 6428 (February 15, 2019), pp. 694-695, DOI: 10.1126/science.aaw5670.
- Matthew Chin, "Researchers create ultra-lightweight ceramic material that can better withstand extreme temperatures," UCLA Press Release, February 14, 2019.
- Bowtie auxetic material demonstration, YouTube Video by "pasemanadmin".