
Diamond Elasticity
July 30, 2018 I have a huge collection of music compact disks (CDs), none of which have been played in the past year. When music CDs were introduced they were much more convenient than phonograph records, and sales of phonograph records steeply declined. Likewise, digital music files are much more convenient than CDs, so it's now hard to find music CDs. I think my last music CD purchase was twenty years ago from an actual brick-and-mortar record store. There are presently no record stores in my area, since nearly all music is procured online. One CD in my music collection is Diamond Music, a 1996 compilation of music by composer, Karl Jenkins.[1] This music was used in a series of diamond gemstone commercials of that time that had the helpful recommendation that a diamond engagement ring should cost two month's salary.[2] The sentiment that Diamonds are Forever was used in those commercials, and this was the title of a much earlier novel by Ian Fleming and a James Bond film. Chemists know that diamonds are actually perishable and not really eternal. The "Diamonds are Forever" phrase stems from the popular notion that diamonds are indestructible. However, diamond is just another carbon allotrope, so it will burn. The Gibbs free energy shows that oxidation is favored even at room temperature; viz.,[3]C(Diamond) + O2(gas) -> CO2(gas)While the negative energy indicates a favorable reaction, an activation energy must be overcome before the reaction initiates. For this reason, diamond will only ignite at a temperature of 850-1,000 °C in air, or 720-800 °C in pure oxygen. In 1772, Antoine Lavoisier used a lens to focus the Sun's rays onto a diamond in an oxygen atmosphere to heat it sufficiently to produce carbon dioxide.
ΔGf(Diamond) = 0.693 kcal/mole
ΔGf(Oxygen) = 0 kcal/mole
ΔGf(Carbon Dioxide) = -94.258 kcal/mole
ΔG(Reaction) = -94.951 kcal/mole
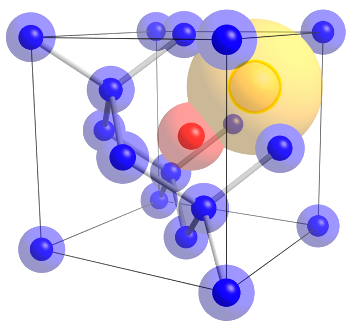
Diamonds are not forever, and they're often imperfect. They can contain atoms other than carbon in their crystal lattice.
This figure shows a nitrogen atom (red) combined with a vacancy (yellow) to form a color center in diamond.
This defect produces a local negative charge that produces a red light photoemission from visible light excitation.
(Wikimedia Commons image by Stacylee14.)
Technology is disrupting many established markets, from taxicabs to television, and it's now disrupting the diamond market. Many of today's young people don't subscribe to the two month's salary diamond, and there are now synthetic diamond gems available for half the price of a mined diamond, $4,000 per carat, rather than $8,000 per carat.[4] Diamond marketer, De Beers, the "Diamonds are Forever" corporation, has always argued that synthetic diamonds are somehow inferior to mined diamonds to justify the higher price of mined diamonds. Seeing how synthetic diamonds are eroding the $80 billion diamond gem market, De Beers has recently announced that it will also supply synthetic diamonds.[4] De Beers is still claiming that synthetic diamonds are inferior, so much so that their synthetics will be sold for about $800 a carat.[4] Many commentators claim that De Beer's sole motivation is to disrupt the synthetic diamond market, as this low price surely will, as well as to underscore the idea of the supposed inferiority of synthetic diamonds. De Beers says that its synthetics will not be graded for color and clarity, as are mined gems, which is apparently another marketing ploy.[4] Synthetic diamonds are often superior to mined diamonds in such grading, since an industrial process can be well controlled and mined diamonds are a product of the vagaries of nature. One of my crystal-growing colleagues was certified as a diamond appraiser by the Gemological Institute of America, possibly as a hedge against diminishing employment among crystal-growers in the US.
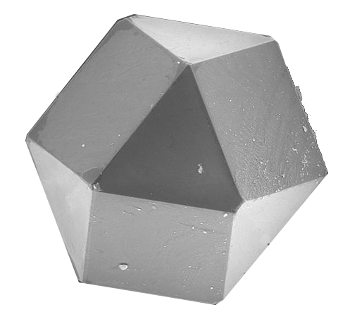
Nature imitating geometry - A scanning electron microscope image of a synthetic diamond cuboctahedron.
A cuboctahedron has 8 triangular faces and 6 square faces. Such a polyhedral shape arises from the different growth rates on different crystal facets.
(Portion of a Wikimedia Commons image by Ludvig14.)
Aside from its use as a gemstone, diamond is a useful industrial material since it has exceptional hardness and strength. The semi-quantitative Moh's hardness scale that ranks minerals by which will scratch which, puts diamond at the top with a Moh's hardness value of 10, since it will scratch every other mineral on the list (talc is the lowest, with a Moh's hardness value of 1). Some of its other mechanical properties are listed in the following table.[5-6]
| Property | Value | Units | Reference |
|---|---|---|---|
| Young's Modulus | 1,210 | GPa | [5] |
| Bulk Modulus | 548 | GPA | [5] |
| Compressive Strength | 20 | GPa | [5] |
| Tensile Strength | 60 | GPa | [6] |
| Melting Point | 4180 | K | [5] |
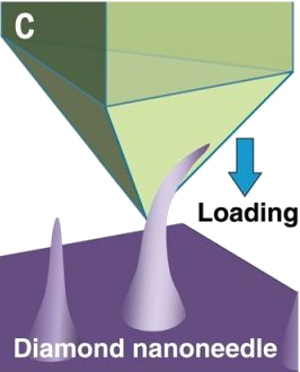
Bending a diamond nanoscale needle in a scanning electron microscope.
(Portion of a Institute for Basic Science (Ulsan, Republic of Korea) image.)
It was found that even polycrystalline diamond nano-needles could achieve a strain of about half the value, 4%, of crystalline needles.[10-11] The researchers ascribe the high strength of all needles to the small number of defects that can exist in a nanoscale needle and the smooth surfaces of the nano-needles.[7,9] Says Feng Ding, an author of this study and a professor at the Institute for Basic Science, "When outside force is applied to these defects, they can crack and eventually break."[9] It was necessary to use a computer model to derive the material stress from the nonlinear elastic deformation for the diamond needles of the particular shape used in the experiments.[10] The model showed that the maximum local stress was close to the theoretical tensile strength of defect-free diamond.[10]
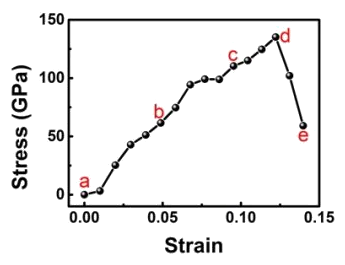
Stress-strain curve for diamond nanoscale needles.
(Portion of a Institute for Basic Science (Ulsan, Republic of Korea) image.)
Not surprisingly, such an unusual material has some potential applications. First, material properties of highly strained materials are often shifted from their quiescent state. This would allow tuning of such things as mechanical, thermal, optical, magnetic, electrical, and light-emitting properties.[11] In particular, the nitrogen-vacancy emission in diamond, noted in a figure above, should shift in wavelength.[11] There may be a means of using strain to permanently encode data in diamonds optically.[11] Since diamond is a biocompatible material, these nano-needles could be used to deliver drugs into cells.[11]
References:
- Karl Jenkins and Adiemus-Palladio 1st Movement from Diamond Music, YouTube Video, January 13, 2009.
- De Beers A Diamond is Forever Commercial (1997), YouTube Video by RetroCommercial.com, March 17, 2011.
- Free energy data from L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- Thomas Biesheuvel, "De Beers to Sell Diamonds Made in a Lab," Bloomberg, May 29, 2018.
- Diamond (C) - Properties, Applications, AZO Material Website.
- R. H. Telling, C. J. Pickard, M. C. Payne, and J. E. Field, "Theoretical Strength and Cleavage of Diamond," Physical Review Letters, vol. 84, no. 22 (May 29, 2000), pp.5160-5163, DOI:https://doi.org/10.1103/PhysRevLett.84.5160.
- Amit Banerjee, Daniel Bernoulli, Hongti Zhang, Muk-Fung Yuen, Jiabin Liu, Jichen Dong, Feng Ding, Jian Lu, Ming Dao, Wenjun Zhang, Yang Lu, and Subra Suresh, "Ultralarge elastic deformation of nanoscale diamond," Science, vol. 360, no. 6386 (April 20, 2018), pp. 300-302, DOI: 10.1126/science.aar4165 .
- Javier LLorca, "On the quest for the strongest materials," Science, vol. 360, no. 6386 (April 20, 2018), pp. 264-265, DOI: 10.1126/science.aat5211.
- UNIST Introduces Novel Method to Grow Elastic Diamonds, UNIST Press Release, April 27, 2018.
- David L. Chandler, "How to bend and stretch a diamond," MIT Press Release, April 19, 2018.
- World's hardest material, diamond, is flexible, Nanyang Technological University Press Release, April 20, 2018.
- Think diamonds are unyielding? Think again, American Association for the Advancement of Science Press Release, April 19, 2018.
- The 4Cs of nanodiamonds, YouTube Video by Nanyang Technological University, April 19, 2018.