
Bio-Inspired Electrode
May 7, 2018 Along with the obligatory calculation of the lengths of the sides of a triangle, students of trigonometry learn how to calculate the areas of simple polygons. The area of simple planar objects, such as circles, triangles, and rectangles has been known for more than two millennia, and Archimedes (c.287 BC - c.212 BC) determined the surface area of a sphere in 225 BC in his manuscript, On the Sphere and Cylinder. It's interesting that finding the surface area of a sphere is a common exercise for students of calculus, an area of mathematics that was developed two thousand years after Archimedes. The usual formula for calculating the area of a triangle is taking half the product of its base and altitude. This, of course, requires first calculating the altitude. That's why I was happy when I discovered Heron's formula for calculating the area of an arbitrary triangle from the length of its sides. I wrote about Heron's formula in a previous article (Random Triangles, January 26, 2017).
Hero of Alexandria (c. 10 - 70 AD), more properly, Heron, was both a mathematician and an engineer, having invented quite a few devices, including a steam engine, called the Aeolipile.
(Illustration from the Codex of San Gregorio de Nizance, a ninth century Greek manuscript, via Wikimedia Commons.)
Hero's simple formula for calculating the area A of an arbitrary triangle from the length of its sides appears in his Metrica (c. 60 AD).[1] Using a, b, and c as the length of the sides, the formula is as follows:
Where the parameter, s, known as the semiperimeter, is half the perimeter; viz.,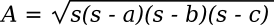
If you want to eliminate the semiperimeter from the formula, the formula can be written in terms of the sides, only, as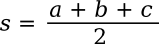
Decades ago, surface area measurement of materials was generally important for just one thing, catalysis. Since heterogeneous catalysis involves the disassociation and reassembly of gas or liquid molecules on the surface of a solid, it's important in research to know how much surface is available for catalysis. There's the additional research goal of trying to maximize the surface area of a particularly good catalyst. One easy way to determine the surface area of a solid is to measure the quantity of an adsorbed gas and relate that to the area occupied by one gas molecule. This method was pioneered by Nobel Laureate Irving Langmuir (1881-1957) who developed what's called the Langmuir isotherm in 1916. Langmuir was awarded the Nobel Prize in Chemistry in 1932 "for his discoveries and investigations in surface chemistry."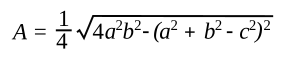
Hitting the books at an early age can lead to a Nobel Prize at a later age, as evidenced by Irving Langmuir (1881-1957).
Langmuir spent much of his working life at the General Electric Research Laboratory, Schenectady, New York, starting there in 1919.
Science fiction author, Kurt Vonnegut (1922-2007), who worked as a publicist at the General Electric Research Laboratory in his youth, said that Langmuir was the inspiration for his fictional inventor of ice-nine (not to be confused with an actual phase of water, ice-IX), that eventually crystallized all the water in the world.
(Left image and right image via Wikimedia Commons, modified for artistic effect.)
Langmuir's simple model of adsorption, which concerned just gas adsorption on a surface, supposed that gas molecules would stick to the surface by chemical adsorption or physical adsorption. He also supposed that the adsorbed film would be one molecule thick. He calculated that at a constant temperature (thus, the isotherm designation) the surface coverage θA would follow the simple expression, θA = P/(P + P0), in which P is the partial pressure of the gas, and P0 is a critical pressure. The graph illustrates how the surface coverage levels off at pressures greater than P0, in this example, 50.
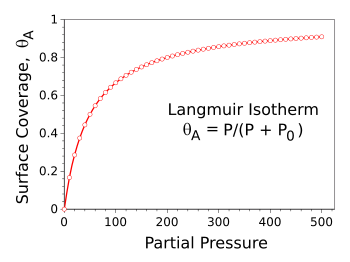
Langmuir isotherm for P0 = 50.
The Brunauer–Emmett–Teller (BET) isotherm is often used in place of the Langmuir isotherm, since it gives better results for physical adsorption of inert gases. It was developed by chemists, Stephen Brunauer (1903-1986) and Paul Hugh Emmett (1900-1985), and physicist, Edward Teller (1908-2003).
It's interesting to note that Brunauer was forced to resign from his position with the US Navy as a consequence of McCarthyism, while his co-author, Teller, was at the opposite end of the political spectrum in his opinion that J. Robert Oppenheimer (1904-1967) should be denied a security clearance.
(Created using Gnumeric. Click for larger image.)
The active surface area of a material has become important in the optimization of electrodes for battery and supercapacitor energy storage. When we examine the relevant equations for a parallel plate capacitor, we see that the stored energy W is directly related to the electrode area A; viz.,
C = κεoA/dwhere C is the capacitance, κ is the dielectric constant, εo is the permittivity of free space (8.854 x 10-12 farads per meter), d is the plate separation, and V is the voltage. With farads as the units of capacitance, the stored energy will have units of joules. Electrode area in electric double-layer supercapacitors is enhanced by the use of very high area electrodes made from activated charcoal. As shown in the figure, a thin dielectric layer is formed between conductive electrolyte layers. This results in many farads of capacitance in a few cubic inches; however, electrochemical decomposition of the electrolyte limits operation to about 3 - 5 volts.
W = (1/2) C V2
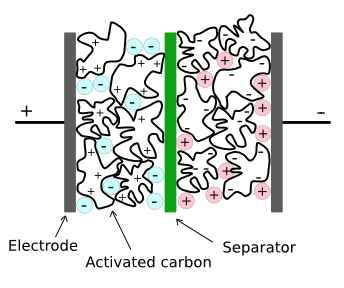
Structure of a conventional supercapacitor.
There are separate ionic liquids that are negatively and positively charged. One disadvantage of current materials is that these ionic liquids decompose at voltages above a few volts. (Via Wikimedia Commons, modified))
Activated charcoal is easy to make, it's inexpensive, and it can be used as an effective high surface area electrode. However, a little more effort can create carbon electrodes of even greater surface area. A team of scientists and engineers from Purdue University (West Lafayette, Indiana), the University of Nevada (Reno, Nevada), Central South University (Changsha, China), Mississippi State University (Starkville, Mississippi), Yangtze University (Jingzhou, China), and the University of California (Los Angeles, California) have developed a bio-inspired leaves-on-branch hybrid carbon nanostructure for supercapacitor electrodes. They've reported their results in an open access paper in Nature Communications.[2-3] Carbon nanomaterials such as carbon nanotubes, nanofibers, and graphene, have been investigated as a way to enhance the energy density of electrodes since they have high surface area and are electrically conductive.[2] Highly-ordered carbon nanomaterials have better properties in electrode applications than randomly-distributed structures, particularly vertical carbon nanotube arrays. Problems with carbon nanotube array electrodes are the poor nanotube bonding to substrates and low tube-to-tube charge transfer efficiency. The nanotube orientation is easily disrupted, resulting in poor mechanical robustness, high internal resistance, and poor cyclic stability, all a consequence of the weak van der Waals forces that bind the nanotubes together.[2] To produce an improved carbon nanoscale electrode architecture, the research team was inspired by tree leaves, which were designed by nature to have a high surface area for carbon dioxide absorption for photosynthesis.[3] Their material design had bio-inspired micro-conduits in which carbon nanotube arrays serve as branches, and graphene plates as leaves.[2] The graphene plates had large surface area, sharp edges, mechanical strength, and high electrical conductivity, all features that are desirable in an electrode material.[2] Says Tim Fisher, principal investigator of this study and a professor of mechanical and aerospace engineering at UCLA,
"We often find inspiration in nature, and plants have discovered the best way to absorb chemicals such as carbon dioxide from their environment... In this case, we used that idea but at a much, much smaller scale - about one-millionth the size, in fact."[3]
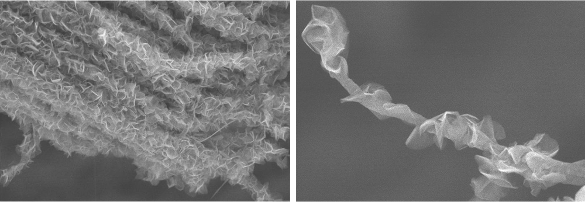
The nanoscale carbon electrode has a branch-and-leaves design incorporating arrays of hollow, cylindrical carbon nanotubes as 'branches,' and sharp-edged, petal-like graphene structures as 'leaves.' (Left image, and right image, from the UCLA Henry Samueli School of Engineering of Applied Science)
The electrode, created by a two-step microwave plasma chemical vapor deposition process, has highly oriented carbon nanotube array walls decorated by graphene.[2] The nanotubes allow a fast diffusion of ions during charge/discharge cycles, while the graphene petals significantly enhance mechanical robustness of the assemblage.[2-3] The hollow, cylindrical carbon nanotubes, are about 20 to 30 nanometers in diameter, and the graphene petal-like structures are about 100 nanometers wide.[3] The tunnel-shaped arrays allow ions to transport stored energy flow with much less resistance between the electrolyte and the surface than if the electrode surfaces were flat.[3] The electrode design provides the same amount of energy storage as similar electrodes, but it's smaller and lighter. Experiments showed that it produced 30 percent better capacitance for its mass than similar carbon materials, and 30 times better capacitance per area. For these reasons it produced 10 times more power and retained 95 percent of its initial capacitance after more than 10,000 charging cycles (see graphs).[3] The areal capacitance was 2.35 Farad/cm2, which works out to be about 500 Farads per gram, and there was a capacitance retention of about 95% over 10,000 cycles.[2-3]
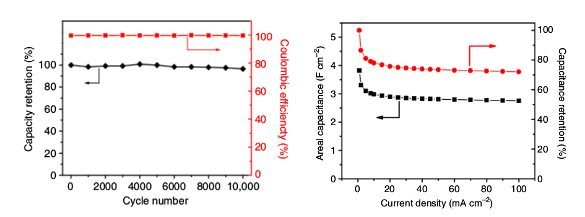
Left, charge/discharge cyclic stability of the electrode at a current density of 60 mA/cm2 and coulombic efficiency. Right, areal capacitance and capacitance retention as a function of current density, as calculated from charge/discharge curves. (Left, fig. 2d, and right, fig. 3d, from ref. 2, licensed under the Creative Commons Attribution 4.0 International License.[2])
It appears that the sharp edges of the graphene increase charge storage and aid the rapid movement of electrolyte ions in the electrodes, thereby leading to improved supercapacitor performance.[2] This research was funded by the US Air Force Office of Scientific Research. The computer simulations were carried out on the High Performance Computing Collaboratory (HPC2) facility at Mississippi State University.[2]
References:
- An English translation of Heron's Metrica is purportedly available as "Codex Constantinopolitanus Palatii Veteris," E.M.Bruins, Editor, vol 3, (Brill, 1964).
- Guoping Xiong, Pingge He, Zhipeng Lyu, Tengfei Chen, Boyun Huang, Lei Chen & Timothy S. Fisher, "Bioinspired leaves-on-branchlet hybrid carbon nanostructure for supercapacitors," Nature Communications, vol. 9 (February 22, 2018), article no. 790, doi:10.1038/s41467-018-03112-3. This is an open access paper with a PDF file available here
- Inspired by nature: Design for new electrode could boost supercapacitors' performance, UCLA Press Release, February 23, 2018.