
Transparent Amorphous Oxide
January 29, 2018 Fake news is not a recent phenomenon. From childhood, I've seen many impossible stories in the tabloids arrayed at supermarket checkouts. Also, several alternative facts were a part of my childhood science education. One of these was the idea that glass was a liquid that continued to flow long after it cooled to room temperature. Glass is not a crystalline solid as are most of the inorganic materials that we encounter daily. Examples of these crystalline solids are metals and table salt. The atoms in crystals are regularly arrayed in a lattice structure, while the atoms in glass are randomly located, in analogy to the atoms in a liquid. So, a glass must be a liquid, right? The cited "proof" of glass being a liquid is that the stained glass panels in centuries-old cathedrals are thicker at the bottom, so the glass flowed in response to gravity just as a liquid would.
Glassmaking started before 3000 BC. As noted in Pliny's Natural History, both furnace technology and glassmaking were extensively developed in the 1st century AD
An image of Hasdrubal Aufdinger, the glaser, from the house book of Landauer Zwölfbrüderstiftung, vol. 1 (Nuremberg 1511-1706).
(Collection of the City Library Nuremberg, via Wikimedia Commons)
As it turns out, the varied thickness of the glass is just an artifact of how the glass was manufactured. The artisans just arranged the thicker part at the bottom by artistic convention. As explained on the website of the Corning Glass Museum, the calculated flow of glass is infinitesimally small.[1] I visited the Corning Glass Museum, which is a three hour's drive from my home town, when I was in high school. This was perhaps one catalyst for my career in materials science. So, where do we place the neither fish nor fowl, neither crystalline solid nor liquid, material called glass? Glass is an amorphous solid, a solid that's a frozen image of a liquid. Metals can be produced as glasses, aptly named metallic glass. One commercially important metallic glass was developed by AlliedSignal (now, Honeywell) and is trademarked as Metglas®. Metallic glasses have magnetic properties that make them suitable for transformer cores and magnetic shielding (see photo).

Metglas® transformer cores.
These cores are the SA1 composition with boron (1-5%), iron (85-95%), and silicon (5-10%).[2]
(photo by the author.)
Metallic glasses closely resemble metals, so they don't possess the transparency that makes most glass valuable. There's a wide range of transparent glass compositions, each developed to serve a particular function. These functions can be as simple as window glazing, or as technically advanced as conductive glass for computer and cellphone displays. The following table gives a few examples of transparent glass compositions.
| Name | Major Components | Uses |
|---|---|---|
| Soda-lime glass | SiO2/Na2O/CaO/Al2O3 | Window glass, bottles |
| Flint glass | SiO2/Na2O/CaO/PbO or BaO | Lenses |
| Crown glass | SiO2/Na2O/CaO | Lenses |
| Borosilicate glass | SiO2/B2O3/Na2O/Al2O3 | labware |
| Aluminosilicate glass | Al2O3/SiO2 | labware |
| Fused quartz | SiO2 | labware |
| ITO | In2O3/SnO2 | Displays |
Toyohashi University of Technology Ph.D. candidate, Takuya Yoshimoto, holding a film of amorphous tantalum-yttrium oxide.
Image copyright © Toyohashi University of Technology, all rights reserved.
Magnetron sputtering was used to fabricate layers of the high refractive index amorphous yttrium-tantalum oxide as part of a dielectric mirror used in an iron garnet magneto-optical device operating at near-infrared wavelengths.[3] The magnetic garnet required thermal treatment at about 750°C.[4] Aside from magneto-optical devices, the tantalum-yttrium oxide material would be useful for spintronic, magnonic, and multiferroic devices as well.[3] Says Taichi Goto, an assistant professor at the Toyohashi University of Technology,
"We used amorphous tantalum yttrium oxide to form a dielectric mirror and combined it with magnetic garnet. Actually, other than magnetic garnet, there are more materials that have not been combined with dielectric mirrors because of the high-temperature thermal treatment that is required. I hope that our findings will help make such materials usable too."[4]
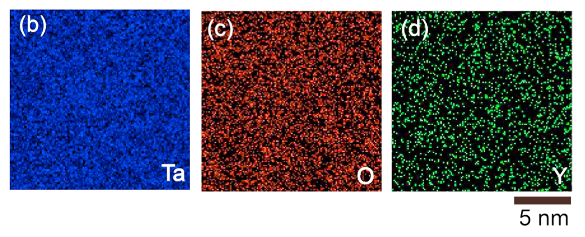
Element maps of tantalum, yttrium, and oxygen in an amorphous Ta-Y-oxide. These maps, created using the EDX technique, demonstrate the amorphous nature of the oxide. (Portion of fig. 5 of Ref. 3, licensed under the Creative Commons Attribution 4.0 International License.)
References:
- Robert Brill, "Does Glass Flow?" The Corning Museum of Glass Web Site, September 29, 2011.
- Material Safety Data Sheet, METGLAS® 2605 SA1 Iron Based Alloy, Metglas Inc. Web Site (PDF File).
- Takuya Yoshimoto, Taichi Goto, Hiroyuki Takagi, Yuchi Nakamura, Hironaga Uchida, Caroline A. Ross, and Mitsuteru Inoue, "Thermally stable amorphous tantalum yttrium oxide with low IR absorption for magnetophotonic devices," Scientific Reports, vol. 7, Article no. 13805 (October 23, 2017), doi:10.1038/s41598-017-14184-4. This is an open-access paper with a PDF file here.
- High-refractive-index material retains high transmissivity after annealing at 850 degrees C, Toyohashi University of Technology Press Release, November 27, 2017.