
Agitated Atoms
August 28, 2017 As one learns very early in elementary school science, temeperature is our way of quantifying the motion of atoms. In a capillary thermometer, filled with mercury in early days, but now filled with ethanol, this movement of atoms causes the liquid to expand, so the liquid rises in the tube. Ethanol is not only safer than mercury for thermometry, but it's more sensitive to temperature, having a volumetric expansion coefficient of 750 ppm/K, compared with the 182 ppm/K of mercury. But, as all will agree, electronic thermometers are now the best for home use. The idea that temperature is related to the movement of atoms goes back to Gay-Lussac's law that the pressure of a gas in a fixed volume is proportional to its absolute temperature. This law is the macroscopic example of the kinetic theory of gases that relates the random motions of individual molecules to pressure. All those small impacts of the molecules on the wall of a container, when added together, result in the pressure that's observed.
Joseph Louis Gay-Lussac (1778-1850).
Gay-Lussac was a French chemist and physicist who co-discovered the fact that water is formed from two parts hydrogen and one part oxygen, and he co-discovered the element, boron.
(Via Wikimedia Commons.
This temperature-induced shaking of atoms in solids is useful in annealing, the process by which an atomic structure "frozen" into a specimen is relaxed and then allowed to enter an equilibrium structure by atomic movement during slow cooling. This process generally results in a softer material that's easier to shape, with strength recovered by a subsequent hardening treatment. Computer science has created useful algorithmic analogies of many natural processes. Foremost among these are artificial neural networks, genetic algorithms, and ant colony optimization, all taken from biology. Another is simulated annealing, obviously taken from metallurgy. One example of simulated annealing is placement of components on a circuit board to minimize connection length. The system "energy" that we wish to minimize is related to the total conductor length, and the "atoms" that move around are the components. Initially, the "temperature" is set so high that things move around at random. As the temperature is decreased, some ideal component placements are more likely to persist, while other components will still bounce around. If the cooling rate is slow enough (and we were careful in our definitions of "energy" and "temperature"), the component placement will freeze into its best state.
Printed circuit board conductor traces.
A familiar sight to many who have built their own desktop computers from components.
(Flickr image by Karl-Ludwig Poggemann, Creative Commons licensed.
Temperature is about movement, which leads to the idea that acoustic vibration can be used as an annealing process in some systems. A team of Japanese scientists from Osaka University (Osaka, Japan) has used this method to crystallize colloids.[1-3] They crystallized a hard-sphere colloidal glass by mechanical oscillation, and they found that accelerated crystallization occurred at a specific oscillation frequency.[1] Crystalline materials are often required for certain applications, so crystal growth has always been an important topic in materials science. Previous research demonstrated that palladium-based metallic glasses could be crystallized below their glass transition temperature by application of ultrasound.[1] It was hypothesized that the natural motion of atoms was stochastically resonant with the motions caused by ultrasonic vibrations.[1] It would be useful if stress-wave agitation can be invoked as an aid to crystallization of amorphous solids.[1] The research team used colloidal glass as a model system, since they are easier to study than normal solids. The phonon (sound wave) modes in actual materials are in the terahertz (THz) range, and this would make experiment difficult.[1] Since hard-sphere colloids have phases similar to those of atomic systems, these were used to facilitate measurement.[2-3] The experiments, as first author of this study, Nobutomo Nakamura, explains, are easy to understand, "We prepared colloidal glasses from silica spheres in solution and then oscillated them at different frequencies... We then observed the resulting structure by confocal laser scanning microscopy."[2-3]
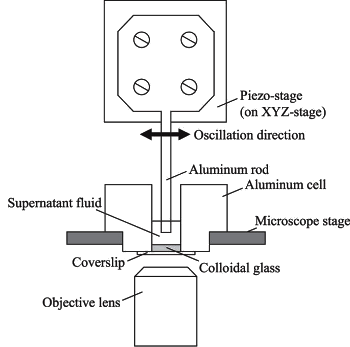
Vibration apparatus for colloid crystallization.
(Fig. 5 of ref. 1, licensed under the Creative Commons Attribution 4.0 International License.)[1
The colloidal glass was formed by centrifugation of silica particles, dispersed in solution, into a sample cell.[1] As can be seen in the above figure, an oscillating square cross section rod with a flat tip generates a shear wave in the supernatant fluid above the colloid by horizontal movement, and this wave propagates to the colloidal glass. There's a 10 micrometer gap between the tip of the oscillating rod and the top surface of the colloidal glass.[1] The oscillating frequency was varied between 30 and 100 Hz with the amplitude set to 5 μm. Images were taken after intervals of five second oscillation, repeating until 60 seconds total oscillation is reached.[1] No crystallization was observed at 70 Hz and below, but crystalline regions were clearly formed at 75 Hz.[1-3] Says Nakamura, "Our results indicated there is a specific vibrational mode that facilitates crystallization of the colloid."[2-3]
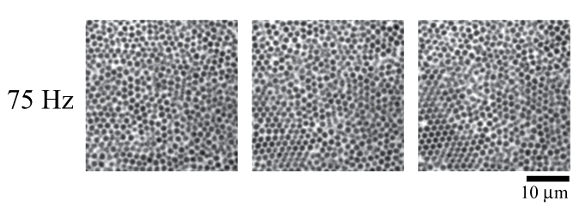
Colloid crystallization by acoustic vibration, in images taken at 0, 30, and 60 seconds. In this experiment, mechanical oscillation at 30 and 70 Hz did not evoke crystallization, but 75 Hz did, as can be seen in the lower left hand corner of the image taken at sixty seconds. (Osaka University images by N. Nakamura, K. Inayama, T. Okuno, H. Ogi, and M. Hirao
To confirm the hypothesis that the crystallization frequency depends on the interaction between particles, the researchers added a polymer to the system to alter the interaction force, and this caused the crystallization frequency to increase.[2-3] These experiments demonstrate that the crystallization of amorphous systems could be aided by agitation at a specific frequency, so it might not always be required to heat them above their glass transition temperature. This would allow formation of crystalline materials at a lower temperature, and this might be useful in device manufacturing.[2-3]
References:
- Nobutomo Nakamura, Kyosuke Inayama, Tasuku Okuno, Hirotsugu Ogi, and Masahiko Hirao, "Accelerated crystallization of colloidal glass by mechanical oscillation," Scientific Reports, vol. 7, Article no. 1369, May 2, 2017, doi:10.1038/s41598-017-01484-y. This is an open access article with a PDF file available at this same URL.
- Control of Material Crystallization by Agitation, Osaka University Press Release, May 2, 2017.
- Control of Material Crystallization by Agitation, Osaka University Press Release, June 8, 2017.