Carbon Nanotube Textile
May 25, 2017
One
childhood memory I have is the visage of a strange little
bridge on the
road between our house and the
downtown area. The bridge crossed the
Mohawk River, a span of just 250 feet. What was strange about that bridge was the pair of short
girder arches on the sides of the otherwise contiguous road surface. This bridge didn't really look like a bridge, and I always thought that the girders were
decorative, or a way to separate the roadway from the
sidewalk. That was before I learned about
truss bridges.
Rather thick
beams would have been required to carry the
weight of a roadway over such a gap. Lesser
materials served the same purpose by changing the
one-dimensional beams into a
two-dimensional structure. Common
I-beams were
riveted together to create a type of truss bridge called a "pony truss" bridge, an inexpensive structure suited for such a short span. It's no surprise that the dominant
geometrical element of a
truss is the
triangle.
As I explained in a
previous article (Non-Brittle Ceramic Structures, November 21, 2014), while a
quadrilateral assembled from equal length pieces can have a variety of
angles, the angles of a triangle are fixed by their lengths. This property leads to the
dimensional stability of structures built from triangles (see figure).
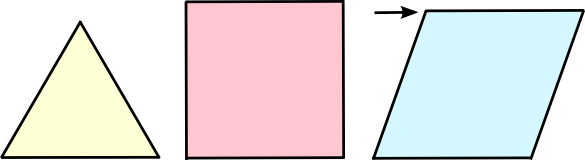 |
| Three equal sides will assemble into only one triangle, but four equal sides can assemble into quadrilaterals of various angles, such as a square (center) and a rhombus (right). The transition from a square to a rhombus is often used as an example of shear. (Created using Inkscape.) |
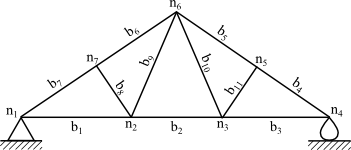
While pony truss bridges are rare, an example of a truss with a long history is probably near where you're seated. In 1839,
Camille Polonceau, a
French railway engineer,
invented the eponymous Polonceau truss, as shown in the figure. This truss is a common element of
roof construction. In honor of his invention, Polonceau's name was among the
72 names engraved on the Eiffel Tower, joining such scientific luminaries as
Cauchy,
Coriolis,
Coulomb,
Gay-Lussac,
Fourier,
Lagrange,
Laplace, and
Lavoisier.
The idea that linear structural elements can be assembled into
strong two-dimensional structures is especially important in the
nanoscale realm.
Multi-walled carbon nanotubes have shown themselves to be individually strong, with
tensile strength about two
orders of magnitude greater than that of
stainless steel.[1] Among other benefits, carbon nanotubes are lighter than
aluminum, and more
conductive than
copper. The utility of these exceptional properties can only be realized in practice when these carbon nanotubes can be joined together to form larger structures.
The problem, of course, is how do you "glue" nanotubes together? A
research team from the
Department of Mechanical Science and Engineering at the
University of Illinois Urbana-Champaign has produced a tough conductive
textile by "capillary splicing" of carbon nanotubes.[2-3] While the term, "capillary splicing," is used for the technique in which things such as
fiberoptic cables are joined through insertion into the separate ends of a
capillary tube, the Illinois technique uses
capillary action in a more general sense. Surfaces will attract each other in order to reduce their
surface energy, the difference in energy between surface
atoms and atoms in the bulk of a
material.
Einstein's first
publication was on capillary action.[4]
As
mobile devices become smaller, there's a push to make them
flexible, as well, but flexure of
electrical conductors is only possible when they resist
fracture. The Illinois team was able to achieve high strength in a nanotube fabric by alignment of the nanotubes on a
substrate, where they couple together through strong
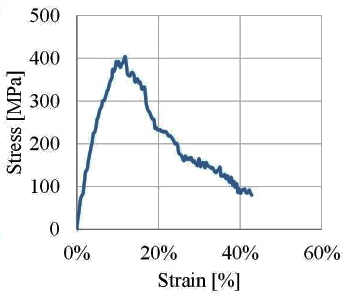
van der Waals forces.[2] the average strength of the as-prepared nanotube fabric was 170
MPa, and its average fracture energy was 16
kJ/
m2, values that are fifty times higher than those for
metal nanofilms.[2]
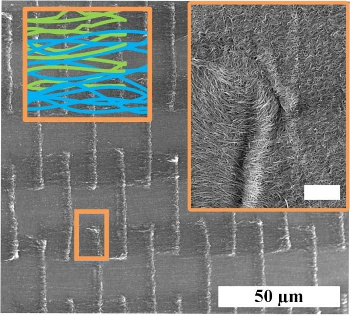
To produce their textile of carbon nanotubes, they first deposited a
catalyst on a
Silica glass substrate. The catalyst was deposited as a staggered pattern not unlike that seen in
brickwork, and in tough
natural materials such as
nacre and
bamboo.[3] Then, vertically-aligned carbon nanotubes were
synthesized by
chemical vapor deposition to produce
parallel lines of 5 μm width, 10 μm length, and 20-60 μm height.[3] This nanotube array was then
coated with
polyvinyl alcohol and
polymethyl methacrylate (PMMA).[3] When the substrate was floated onto
water, the PMMA carrier film was released.[2]
acetone was used to dissolve the PMMA after this film was mounted for
tensile testings to produce a bare nanotube textile.[2]
Says
Sameh Tawfick, an
assistant professor of mechanical science and engineering at Illinois,
"To our knowledge, this is the first study to apply the principles of fracture mechanics to design and study the toughness of nano-architectured CNT textiles. The theoretical framework of fracture mechanics is shown to be very robust for a variety of linear and non-linear materials... Flexible electronics are subject to repeated bending and stretching, which could cause their mechanical failure. This new CNT textile, with simple flexible encapsulation in an elastomer matrix, can be used in smart textiles, smart skins, and a variety of flexible electronics."[3]
Partial
funding for this research came from the
Office of Naval Research.[2]
References:
- Mechanical properties of carbon nanotubes Web Page on Wikipedia.
- Yue Liang, David Sias, Ping Ju Chen, and Sameh Tawfick, "Tough Nano‐Architectured Conductive Textile Made by Capillary Splicing of Carbon Nanotubes," Advanced Engineering Materials, Early View Article (March, 2017), DOI: 10.1002/adem.201600845.
- Rick Kubetz, "Wonder material? Novel nanotube structure strengthens thin films for flexible electronics," University of Illinois Urbana-Champaign Press Release, April 21, 2017.
- Albert Einstein, "Folgerungen aus den Capillaritätserscheinungen," Annalen der Physik, vol. 309, no. 3 (1901), pp. 513-523, doi:10.1002/andp.19013090306. A page image file can be found here.