Graphene from Ethylene
June 5, 2017
As a young
scientist in
elementary school, most of my
scientific knowledge came from
library books, the
newspaper, and such
magazines as
Life and
Popular Science. It was in Popular science that I read how some
fruits could be
ripened by exposure to
ethylene gas. This ripening process works only on
climacteric fruits, such as
tomato,
apple,
melon and
banana, and it doesn't work on non-climacteric fruits such as
citrus,
grapes, and
strawberries.
While some might consider this process to be another insidious way for
corporate agriculture to fool both us and
Mother Nature, we would not have such a variety of fruits available to use without it. Using this process, fruits can be
harvested before ripening,
shipped long distances, and appear at our local
supermarket in a form that we enjoy
eating. This is most apparent for bananas, which need to travel extreme distances to reach
Tikalon's Northern New Jersey home, so they're picked while still green and exposed to ethylene somewhere in transit.
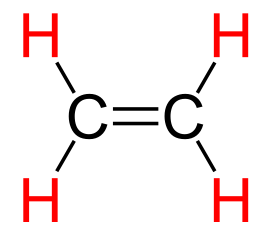
As you likely guessed, ethylene is the
reagent for making
polyethylene. More than half of ethylene production is used to make polyethylene, the most widely used
polymer.
High-density polyethylene (HDPE), a
material used in many of my
grandchildren's toys, has a high
ratio of
strength to
density. This arises from the low degree of
branching in the polyethylene polymer that results in larger
intermolecular forces between the
polymer chains. HDPE is commonly used in
food storage containers,
plastic bottles, and
Tyvek barrier films used in
construction.
Graphene, a material formed as a monolayer of
carbon atoms, has been a popular
nanoscale material to
research in the last few
decades. Its potential as a useful
electronic and
structural material is underscored by the short span of time between its discovery and the award of a
Nobel Prize to its discoverers,
Andre Geim and
Konstantin Novoselov. These physicists first
published a paper on graphene in 2004, and they received the 2010
Nobel Prize in Physics.
I've written quite a few articles on this wonder material, the most recent of which can be viewed
here (Soybean Graphene, March 23, 2017). That article discussed a novel technique for graphene
synthesis developed by
Australian scientists from
CSIRO Manufacturing (Lindfield, New South Wales, Australia), the
University of Sydney (Sydney, Australia), the
University of Technology (Sydney, Australia), and
Queensland University of Technology (Brisbane, Australia). Their process used
soybean oil as a
precursor to graphene.
While graphene is typically grown using
chemical vapor deposition techniques, an international research team with members from the
Technische Universität München (Garching, Germany), the
University of St. Andrews (St. Andrews, United Kingdom), and the
Georgia Institute of Technology (Atlanta, Georgia) has examined production of graphene by assembly of
adsorbed molecules of ethylene on the (111)
crystal facet of
rhodium. They report their results in an
open access article in the
Journal of Physical Chemistry C.[1-3]
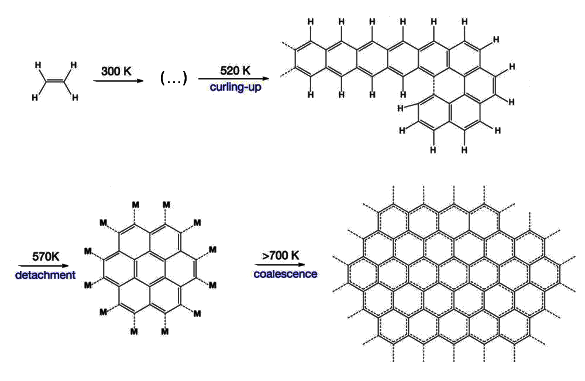
This graphene synthesis technique is inspired by the common process of
coking in which
organic compounds will transform to carbon when adsorbed onto
metal surfaces.[1] While coking is important in
steelmaking, it's also a nuisance in
catalysis, since it
poisons the catalytic surface.[1] The present process utilizes this coking process on a catalyst in which heated
one-dimensional polyaromatic hydrocarbons (1D-PAH) are converted into
two-dimensional molecules.
Surface diffusion allows
coalescence of these molecules into graphene.[1]
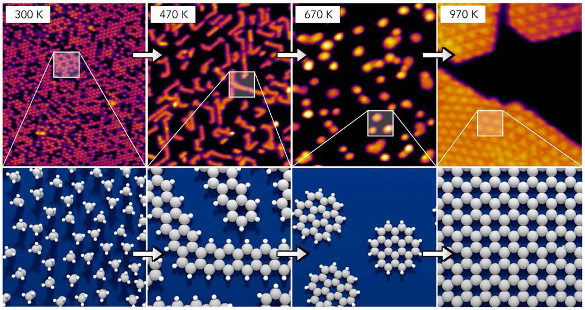
Some earlier efforts to produce graphene from simple
hydrocarbon precursors created
soot, as would be expected in a coking process, rather than graphene.[2] The trick was to heat the ethylene in stages, finally to a higher temperature than before. This resulted in pure layers of graphene on the rhodium surface.[2] As ethylene lost
hydrogen atoms, the remaining carbon atoms
self-assembled into the
honeycomb bonding of graphene.[2]
On initial heating above room temperature, the ethylene links into one-dimensional
chains of polyaromatic hydrocarbons. Additional heating caused these chains to
crosslink into two-dimensional molecules that surface-diffuse and coalescence into high purity graphene.[2] As
Uzi Landman, a
professor of
physics at the Georgia Institute of Technology who led the
theoretical component of this research,
"The temperature must be raised within windows of temperature ranges to allow the requisite structures to form before the next stage of heating... If you stop at certain temperatures, you are likely to end up with coking."[2]
To understand the process, the research team used
scanning-tunneling microscopy,
high-resolution electron energy loss spectroscopy, and
thermally programmed desorption to observe and characterize the surface components at each process step.[2]
Dehydrogenation was an important step, but not all hydrogen is removed at once. Some of the hydrogen remains, and it aids the bond-breaking process that detaches the larger molecule precursors and allows them to become mobile.[2]
While this graphene synthesis technique is simple and potentially a lower
cost alternative to chemical vapor deposition, the problem remains that the graphene is attached to the rhodium
substrate and must be removed.[2] This research was funded by the
Air Force Office of Scientific Research and the
Office of Basic Energy Sciences of the
U.S. Department of Energy.[2]
References:
- Bo Wang, Michael König, Catherine J. Bromley, Bokwon Yoon, Michael-John Treanor, José A. Garrido Torres, Marco Caffio, Federico Grillo, Herbert Früchtl, Neville V. Richardson, Friedrich Esch, Ueli Heiz, Uzi Landman, and Renald Schaub, "Ethene to Graphene: Surface Catalyzed Chemical Pathways, Intermediates, and Assembly," J. Phys. Chem. C, vol. 121, no. 17 (March 14, 2017), pp. 9413-9423, DOI: 10.1021/acs.jpcc.7b01999. This is an Open access article with a PDF version available here.
- John Toon, "High Temperature Step-by-Step Process Makes Graphene from Ethene," Georgia Institute of Technology Press Release, May 4, 2017.
- Scanning-tunneling microscope video of ethylene decomposition at 455 K and subsequent polyaromatic hydrocarbon formation.