Freezing, Outside-In
March 9, 2017
While my
childhood was influenced in many ways by
Walt Disney's creations, I now try to avoid all things
Disney, since I object to that company's advocacy of
perpetual copyright. As a child of the
1950s, I watched Disney's
Davy Crockett, and the
Mars exploration episodes of
Walt Disney Presents. I also enjoyed watching
Annette Funicello , a native of my home town of
Utica, New York, on
The Mickey Mouse Club.
One
Christmas ritual of my
family is our watching an old
VHS tape of Christmas-themed Disney
cartoons entitled, "A Walt Disney Christmas." The earliest cartoon on this tape is the 1932
Santa's Workshop. Although I found a copy of this cartoon on
YouTube, I believe that it's still under
copyright after all these years. The term for a 1934 copyright, barring other circumstances, is presently 95 years. The cartoon is notable in the unusual sense that it contains fleeting
racial and ethnic stereotypes, most of which have been removed in later releases.
The Disney
animated films, especially the so-called "
Disney Princess" films are wildly popular, since they entertain both
children and their
parents and
grandparents. There's been an attempt to
recast these films to make them attractive to
boys in addition to
girls. Thus, recent films such as the 2010
Tangled and 2013
Frozen, aren't titled after a female character, and they feature many action sequences.
While the
magical aspects of Frozen are
fiction, there is some respect for the
physics of
ice and the
freezing of
water; notably, the idea that ice can be created out of water in the
air to blanket the
Kingdom of Arendelle. However, as pointed out in a
paper by Aaron Goldberg of
McMaster University, the physics of this movie fails when you consider the
energy required to freeze all that water.[1]
Since the
Hans Christian Andersen "
Snow Queen"
fairy tale on which the movie is based has the
Norwegian fjord,
Nærøyfjord, as the frozen locale, Goldberg calculated that about 10
11 kilograms of water are involved. A
Carnot cycle refrigerator, the most
efficient heat engine, would require 5.8 x 10
15 joules to freeze that much water into ice. This is about a hundred times the energy released in the
Hiroshima atomic bomb. Writes Goldberg,
"This immense number puts Elsa's power into perspective, implying either that the Snow Queen has enormous strength, or that Disney underestimated the ramifications of their animated fantasy."[1]
The Snow Queen is one
feminist that you wouldn't want to
rile!
It comes as no surprise to a
crystal grower like me that ice tends to
nucleate on atmospheric particles, since large,
technologically useful,
crystals are grown on smaller
seed crystal substrates. The world's
deserts inject about 10
12 grams of
dust into the atmosphere annually.[2] Atmospheric water can exist for days in a
supercooled state at
very low temperatures, even below -33
°C, without changing into ice, but a few particles of dust can transform a
cloud of water to ice.[3]
A
research team from the
Karlsruhe Institute of Technology (Eggenstein-Leopoldshafen, Germany),
University College London (London, UK), and the
Universität Heidelberg (Heidelberg, Germany) has recently published a study of ice nucleation on
potassium-feldspar, KAlSi
3O
8, a common
mineral dust present in the atmosphere.[2-4]
Feldspar is unusually effective as a substrate for ice nucleation, and this study showed that ice preferentially nucleates at a high rate on
surface defects on the feldspar. Nucleation was found to be most favorable on the
(100) crystal planes of feldspar, which are not exposed as
facets on natural feldspar. This plane, however, is exposed at steps, cracks, and cavities on other facets.[2-3]
Water is unusual among
liquids, since it expands when it solidifies to ice, rather than shrink as other liquids do when they solidify. Ice is about 9% larger in
volume than the water from which it forms, since ice consists of a
hydrogen-bonded array of water
molecules, and this forced arrangement of the molecules results in a larger volume.
A team of
scientists from the
University of Twente (Enschede, the Netherlands),
Tsinghua University (Beijing, China), and the
Max Planck Institute for Dynamics and Self-Organization (Göttingen, Germany) has just published a study in which they looked at the consequences of this expansion on the
dynamics of a
drop of water freezing from the outside-in.[5] This study uses
high speed video analysis to show how the self-confinement of the expanding inner
solid by the outer
rigid ice shell is resolved.
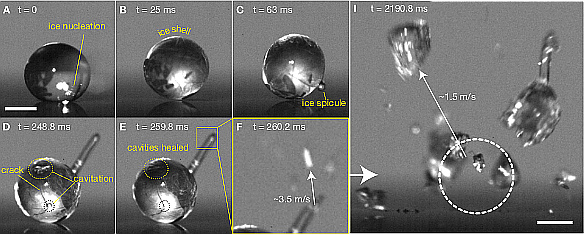
Previous studies have revealed two interesting features of this process. First, an ice
spicule appears on the droplet surface; and, second, the droplets
explode and fly apart into pieces. Such explosions can explain the shapes of the larger ice particles in
clouds and
hailstone nuclei.[5].
To perform these
experiments, the research team placed small droplets of clean,
degassed water on a
glass slide, coated with a
super-hydrophobic layer of
candle soot, in a
vacuum chamber. This allowed
spherical droplets to cool by
evaporative cooling in vacuum, and a final supercooled temperature of about -7°C was achieved by placement and control of
thermal reservoirs. The final chamber
pressure was 340 ± 10
Pa, which is the
equilibrium vapor pressure at that temperature. Ice nucleation was accomplished by touching with the tip of
silver iodide crystal. Silver iodide crystals are
lattice-matched to ice, and this explains their ability to rapidly nucleate ice.[5]
Details of droplet freezing are seen in the above figure. The ice shell forms in a few tens of
milliseconds, then a frozen spicule is ejected, forced out by the increased pressure. The spicule grows in length to about a droplet
diameter, and when it stops growing, the internal droplet pressure increases. The droplet shell undergoes numerous cracking and crack-healing cycles; and, finally, after two seconds, the droplet explodes.[5]
The research team developed a
mathematical model of the process based on the
material and
thermophysical properties of ice. The model shows that the fragment velocity for
millimeter-sized droplets is
independent of the droplet size and depends only on the
bulk modulus of water and the
tensile stress of the ice.
Surface tension becomes important for smaller droplets, and it's predicted that water droplets of radius below 50
micrometers won't explode.[5]
References:
- Aaron Goldberg, "Powering Disney's Frozen with a Carnot refrigerator," Journal of Interdisciplinary Science Topics, vol. 3, February 19, 2014. A PDF file can be found here.
- Alexei Kiselev, Felix Bachmann, Philipp Pedevilla, Stephen J. Cox, Angelos Michaelides, Dagmar Gerthsen, and Thomas Leisner, "Active sites in heterogeneous ice nucleation—the example of K-rich feldspars," Science, vol. 355, no. 6323 (January 27, 2017), pp. 367-371, DOI: 10.1126/science.aai8034.
- Benjamin J. Murray, "Cracking the problem of ice nucleation," Science, vol. 355, no. 6323 (January 27, 2017), pp. 346-347, DOI: 10.1126/science.aam5320.
- Understanding how ice crystals form in clouds, London Centre for Nanotechnology, January 27, 2017.
- Sander Wildeman, Sebastian Sterl, Chao Sun, and Detlef Lohse, "Fast dynamics of water droplets freezing from the outside-in," arXiv, January 24, 2015.