
Ammonia Synthesis
March 6, 2017 Ammonia is an important industrial chemical, with nearly 200 million tons being produced annually. About 14 million tons of ammonia are used to make nitrate fertilizers,[1] and the rest is used for such industrial applications as nitriding of metals, neutralization of acids and stack emissions, manufacture of nitric acid for the synthesis of a variety of chemicals. As anyone who's ever painted with latex paint knows, ammonia is used also to stabilize latex to prevent premature coagulation. As a consequence of its low boiling point (-33.34 °C, -28.0 °F) and high enthaly of vaporization (23.35 kJ/mol, 5.56 kcal/mol at its boiling point), ammonia is used as an industrial refrigerant for the preservation of food, beverages, and as a way to freeze skating rinks. For comparison, water has an enthaly of vaporization of 40.68 kJ/mol, or 9.72 kcal/mol at its boiling point. Because of its toxicity, you won't find ammonia in your home refrigerator, but home refrigerators using ammonia or the likewise toxic gases, methyl chloride and sulfur dioxide, were used about a century ago. The hazards of such toxic refrigerator gases inspired Einstein and his friend, Leo Szilard, to invent a safe alternative. As Szilard related to MIT physicist, Bernard Feld, Einstein read how an entire family, the parents and several children, had been killed by leaking refrigerator fumes as they slept.[3] Spurred by this tragedy, the "Einstein refrigerator" was invented and patented.[4] While this refrigerator still used the standard gases, it was safer since it had no moving parts and did not require rotary seals (see figure).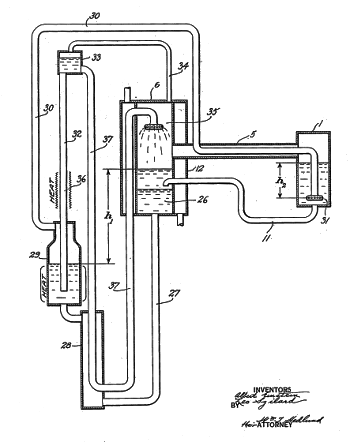 | Figure from US Patent No. 1,781,541, "Refrigeration," by Albert Einstein and Leo Szilard, dated November 11, 1930. The "Einstein refrigerator" is an absorption refrigerator in which the cooling evaporate is absorbed by another liquid, which is run through a heat exchanger to recover the refrigerant liquid for another refrigeration cycle. (Via Google Patents.[4]) |
 |
| Ammonium chloride (sal ammoniac), as mentioned in Pliny's Natural History, Book XXXI, Chapter 39, line 79.[5] My translation of this reads, "For example, the region of Cyrenaica is notable, also, for hammoniacum, which is found beneath the sands. It is similar in color to the alum which is called schiston, it consists of long masses, not transparent, has a foul taste, but is useful as medicine." (Simulated manuscript created using GIMP.) |
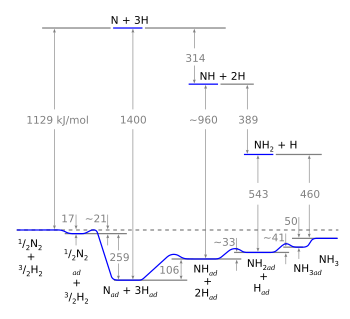 | Why we need a catalyst. Breaking nitrogen and hydrogen molecules into their constituent atoms takes a lot of energy, but all this energy is recovered in the end. (Wikimedia Commons image by Marsupilami.) |
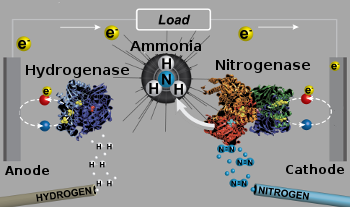 | Schematic diagram of a bioelectrochemical hydrogen-nitrogen fuel cell. (Modified University of Utah image by Ross Milton.) |
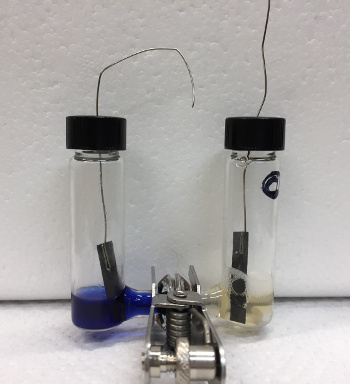 | Working hydrogen-nitrogen fuel cell. Carbon paper electrodes are used for the anode (left) and cathode (right). Methyl viologen is used as an electron carrier in each half-cell. (University of Utah image by Ross Milton.) |
"It's a spontaneous process, so rather than having to put energy in, it's actually generating its own electricity."[8]
References:
- World Fertilizer Trends and Outlook to 2018, Food and Agriculture Organization of the United Nations, Rome, 2015 (PDF File).
- Ammonia Data Page, Wikipedia.
- Gene Dannen, The Einstein-Szilard Refrigerators, Scientific American, January 1997, pp. 90-95.
- Albert Einstein and Leo Szilard, "Refrigeration," US Patent No. 1,781,541, November 11, 1930.
- Pliny the Elder, "Naturalis Historia," Book 31, Chapter 39, on the University of Chicago Penelope web site by Bill Thayer.
- Venkat Pattabathula and Jim Richardson, "Introduction to Ammonia Production, American Institute of Chemical Engineers, September, 2016.
- Ross D. Milton, Rong Cai, Sofiene Abdellaoui, Dónal Leech, Antonio L. De Lacey, Marcos Pita, and Shelley D. Minteer, "Bioelectrochemical Haber–Bosch Process: An Ammonia-Producing H2/N2 Fuel Cell," Angewandte Chemie International Edition, February 3, 2017, DOI: 10.1002/anie.201612500.
- Paul Gabrielsen, "Flipping the Switch on Ammonia Production," University of Utah Press Release, February 3, 2017.