
VOC Detection with Nanocrystals
February 23, 2017 Modern electronic sensors have easily duplicated the functions of the human senses of sight, hearing, and touch, but they have had a harder time with taste and smell (more properly termed, olfaction). Microwave-oven sized equipment such as infrared spectrometers, mass spectrometers, and gas chromatographs are able to act somewhat as taste and smell sensors. As I wrote in a previous article (Breath Analysis, December 7, 2015), the human sense of smell is not that sensitive, and it's insensitive to certain gases. Most important among these is the poisonous gas, carbon monoxide, that might accumulate in dwellings that use combustion for heating. While we might think that we can smell natural gas, what we smell is actually tert-Butylthiol (t-butyl mercaptan). As any organic chemist will tell you, mercaptans have a strong odor in even small quantities. There's been some research on "electronic noses" for detection of particular gases, especially carbon monoxide and flammable gases such as hydrogen. Some odor sensors detect the weight change of adsorbed chemicals on an analyte-covered quartz crystal or MEMS resonant cantilevers, but carbon monoxide and flammable gas sensors use a much simpler approach of changing electrical conductivity in heated tin dioxide (SnO2, stannic oxide). A good review of other oxides for gas sensing is found in ref. 1.[1]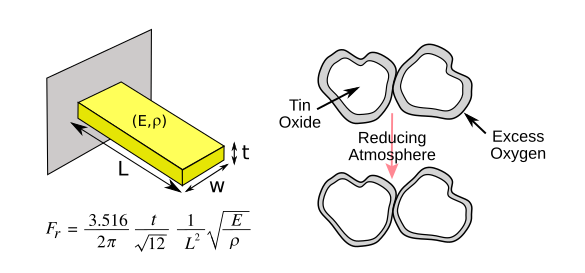 |
| Two styles of electronic noses: A resonant cantilever (left) can be used to detect weight change of adsorbed molecules, while a compressed powder of tin dioxide, when heated, (right) will change resistance when exposed to a reactive gas such as hydrogen because of changes in composition of the particle surface. The cantilever resonance frequency, Fr, depends on the beam thickness t, the beam length L, the Young's modulus E, and the material density ρ. The average density will change when odor molecules are adsorbed. Surprisingly, the width of the beam doesn't figure into the resonance formula. (Created using Inkscape.) |
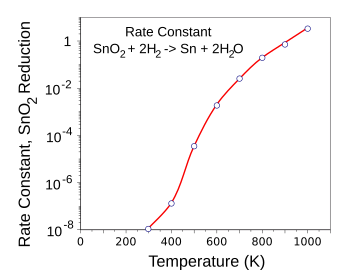 | Rate constant of stannic oxide reduction by hydrogen. At 650 K (375 °C), about one percent of SnO2 is reduced to Sn. (Graphed with Gnumeric from a calculation using free energy data in ref. 2.)[2] |
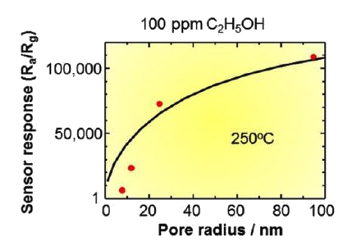 | Tin dioxide nanocrystal sensor response to ethanol as a function of pore size. There's a five orders of magnitude resistance change at 250 °C. (Kumamoto University image by Tetsuya Kida.) |
"Our experiments show that nanocrystal sensors with larger pore sizes gave the best sensor responses. In particular, we found ultra-high sensitivity (increasing by five orders of magnitude) in the devices with largest pore size, the long nanorod sensors... This tells us that is beneficial to have precise control over the manufacturing methods of these types of sensors."[5]Sensing of VOCs is important, since they are an environmental health hazard. Their detection can be used in medical diagnostics, since several VOCs are known biomarkers for glucose malabsorption, alcohol intoxication, and diabetic ketoacidosis.[5] These sensors have capability for alcohol breathalyzer use for alcohol detection at the lower parts-per-billion level.[5] One problem of these SnO2 VOCs sensors is their long recovery time. While they respond to gases within 15-21 seconds, when the gases are removed, they don't return to their normal state for several hours.[5] This appears to be a consequence of reaction residues remaining on the surface. Furthermore, the ethanol sensors saturate when the pore size is greater than 80 nm.[5] However, there are many processing variations to explore to mitigate such effects.
References:
- Chengxiang Wang, Longwei Yin, Luyuan Zhang, Dong Xiang, and Rui Gao, "Metal Oxide Gas Sensors: Sensitivity and Influencing Factors," Sensors, vol. 10, no. 3 (March 15, 2010), pp. 2088-2106, doi:10.3390/s100302088. This is an open access article with a PDF file available here.
- C. E. Wicks and F. E. Block, "Thermodynamic Properties of 65 Elements - Their Oxides, Halides, Carbides, and Nitrides," U. S. Bureau of Mines Bulletin 605, U. S. Government Printing Office (1963).
- Hui Huang, O. K. Tan, Y. C. Lee, T. D. Tran, and M. S. Tse X. Yao, "Semiconductor gas sensor based on tin oxide nanorods prepared by plasma-enhanced chemical vapor deposition with postplasma treatment," Applied Physics Letters, vol. 87, no. 16 (October, 2005), DOI: http://dx.doi.org/10.1063/1.2106006.
- Tetsuya Kida, Koichi Suematsu, Kazuyoshi Hara, Kiyoshi Kanie, and Atsushi Muramatsu, "Ultrasensitive Detection of Volatile Organic Compounds by a Pore Tuning Approach Using Anisotropically Shaped SnO2 Nanocrystals," ACS Appl. Mater. Interfaces, vol. 8, no. 51 (November 30, 2016), pp. 35485-35495, DOI: 10.1021/acsami.6b13006.
- Highly sensitive gas sensors for volatile organic compound detection, Kumamoto University Press Release, February 1, 2017.