
The Voltage Standard
February 25, 2016 Having done much electronic circuit design and construction in my career, I have several multimeters in my home workshop. The multimeters can be configured as voltmeters, I have a few analog voltmeters on my shelves, and my oscilloscope will also function as a crude analog voltmeter. When wired to a voltage source, these will read nearly the same value of voltage, but not the same value. Fortunately, my work doesn't require too precise values of voltage, so I'm not that concerned. One of my digital multimeters will read voltage values to four-and-a-half digits; which, in electrical engineering parlance, means that it will read from 0 to 1.9999 volts. Seeing such a nice array of digits makes you think that your knowledge of voltage is very good. This reminds me of a psychology experiment done many decades ago on how people think that pocket calculators are infallible. A team of psychologists modified calculators to give the wrong answers. As I remember, nearly all the test subjects believed that the calculator answers were right, even though a moment's reflection or a back-of-the-envelope calculation would have indicated that something was amiss. When your laboratory voltmeter shows many digits, how many of these can you believe? Although manufacturers take care to ensure that their computer chips give the right answers, errors will occur. One famous error was the Intel Pentium P5 floating point division bug. This was a subtle error, since it would occur in just one in nine billion divisions. Even then, the error was at the few tens of parts-per-million level. Intel recalled all the faulty chips at a cost of nearly half a billion dollars. I recall that in the interim there was a software patch for the Excel spreadsheet that corrected this error.  |
| (Webcomic xkcd no. 217 by Randall Munroe, licensed under a Creative Commons Attribution-NonCommercial 2.5 License. Click for original on his xkcd web site.) |
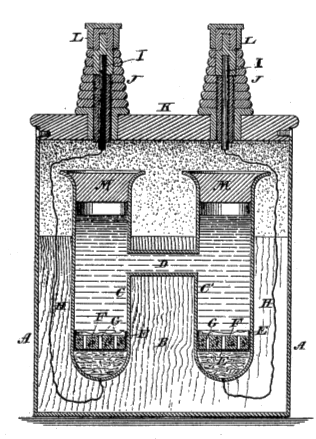 | Fig. 1 of US Patent No. 494,827, "Voltaic Cell," by Edward Weston, April 4, 1893. This cell served as a voltage standard as late as 1990, but it had the disadvantage that it contained the toxic elements, mercury and cadmium. The voltage of a carefully maintained Weston cell is 1.018638 volts, and the temperature coefficient of the voltage is very small. (Via Google Patents.)[2] |
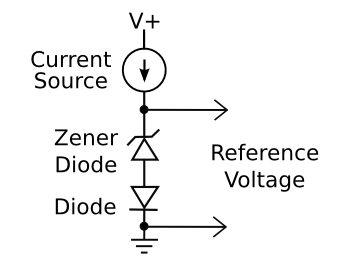 | In the days before bandgap voltage reference circuits, analog designers would combine a Zener diode with an ordinary silicon diode to create a temperature-compensated voltage reference. (Created with Inkscape.) |
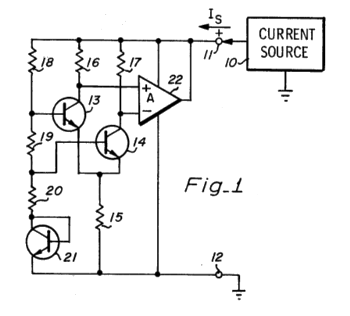 | Fig. 1 of US Patent No. 4,447,784, "Temperature compensated bandgap voltage reference circuit" by Robert C. Dobkin. Dobkin, co-founder of Linear Technology Corporation, has more than a hundred patents. (Via Google Patents.)[3] |
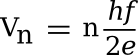 |
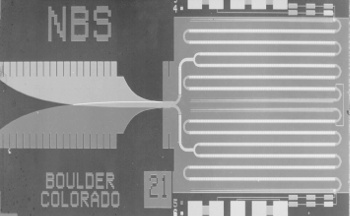 | An array of 3020 superconducting Josephson junctions that act as a voltage reference at liquid helium temperature. (NIST image, via Wikimedia Commons.) |
References:
- Walter J. Hamer, "Thermodynamics of Standard Cells of the Saturated Cadmium Sulfate Type," Journal of Research of the National Bureau of Standards A - Physics and Chemistry, vol. 76A, no.3 (May- June, 1972).
- E. Weston, "Voltaic Cell," US Patent No. 494,827, April 4, 1893.
- P. Brokaw, "A simple three-terminal IC bandgap reference," IEEE Journal of Solid-State Circuits, vol. 9, no. 6 (December 1974), pp. 388–393.
- Robert C. Dobkin, "Temperature compensated bandgap voltage reference circuit," US Patent No. 4,447,784, May 8, 1984.