
The Cheerios Effect
August 25, 2016 If mothers told their young physicists not to play with their food, the youngsters weren't listening. Quite a few discoveries have been made through observations of food items. I've written about several on this blog, including the packing of M&M candy (Packing, November 30, 2010), the Brazil nut effect (Rivers of Sand, December 22, 2014), and wine "legs" (Oenodynamics, November 29, 2011). The unexpected discovery about the lens-shaped M&M candies is that their random packing fills space to 68%, while a random packing of spheres achieves just 64%. Ellipsoids of a different aspect ratio than the M&M candies were found to fill space 78% in random packing.[1] The well-known Brazil nut effect is the tendency for large nuts to move to the top of a container of mixed nuts after shaking. While noticed in cans of mixed nuts, this phenomenon happens in any vertically-shaken, dry, granular mixture of large and small particles.[2-3]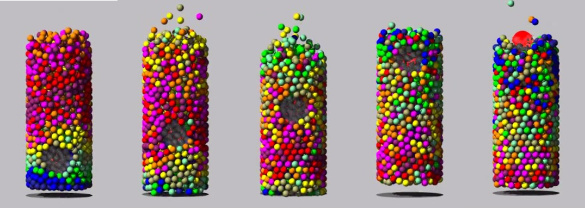 |
| Screenshots of a simulation of the Brazil nut effect. Vibration in the vertical direction causes the large object to rise in a sea of smaller objects. (Used with permission from the Granular Dynamics Update Web Site of Prof. Derek C. Richardson of the University of Maryland - College Park. For further information, see ref. 9) |
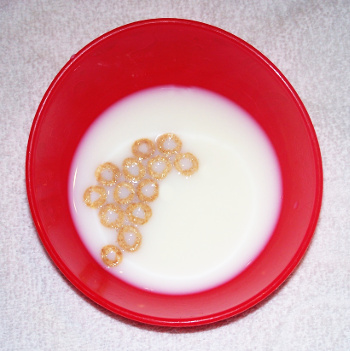 | A demonstration of the Cheerios effect. The presence of the central hole is irrelevant to the attraction, but it aids in keeping the individual pieces afloat. (Photo by author.) |
 | Cheerios were always made from whole-grain oats, but wheat is a common contaminant of oats. General Mills, manufacturer of Cheerios, now certifies it to be gluten-free. (Photo by Conrad Irwin, via Wikimedia Commons.) |
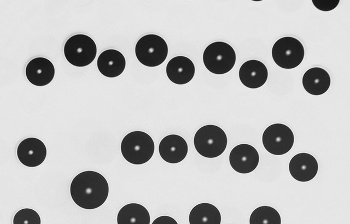 | Still image from a video of water droplets moving on a gel substrate. (Queen Mary University of London image by S. Karpitschka.) |
"...The physical phenomena we have highlighted in this paper suggest ways to design surfaces that prevent fogging or control heat transfer; for instance to create car windows that are always transparent despite high humidity or surfaces that improve heat management in conditioners or boilers. By making surfaces softer or harder, and changing the thickness of the soft layer, we will be able to control how the drops coalesce and spread on the substrate."[8]
References:
- Aleksandar Donev, Ibrahim Cisse, David Sachs, Evan A. Variano, Frank H. Stillinger, Robert Connelly, Salvatore Torquato and P. M. Chaikin, "Improving the Density of Jammed Disordered Packings Using Ellipsoids," Science, vol. 303, no. 5660 (February 13, 2004), pp. 990-993.
- Heinrich M. Jaeger, "Why does shaking a can of coffee cause the larger grains to move to the surface?" Scientific American, March 24, 2003.
- M. Bose, U. U. Kumar, P. R. Nott, and V. Kumaran, "Brazil nut effect and excluded volume attraction in vibrofluidized granular mixtures," Phys. Rev. E, vol. 72, Article No. 021305, August 25, 2005.
- D. Vella and L. Mahadevan, "The 'Cheerios effect'," Am. J. Physics, vol. 73, p. 817, http://dx.doi.org/10.1119/1.1898523. Also at arXiv.
- Eric Forgostona, Leo Hentschkerb, Siobhan Soltaua, Patrick Truitta, and Ashwin Vaidya, "Thermally induced aggregation of rigid spheres on a liquid surface," Physics Letters A, vol. 380, nos. 1–2 (January 8, 2016), pp. 227-231.
- Eric Betz, "A well-known effect in breakfast cereal helps physicists understand the universe," Phys.org, September 9, 2010.
- Stefan Karpitschka, Anupam Pandey, Luuk A. Lubbers, Joost H. Weijs, Lorenzo Botto, Siddhartha Das, Bruno Andreotti, and Jacco H. Snoeijer, "Liquid drops attract or repel by the inverted cheerios effect," Proceedings of the National Academy of Sciences, Early Edition, doi: 10.1073/pnas.1601411113. A PDF file is available here, and a version is available on arXiv.
- Cereal science: how scientists inverted the Cheerios effect, Queen Mary University of London Press Release, June 14, 2016.
- Soko Matsumura, Derek C. Richardson, Patrick Michel, Stephen R. Schwartz, and Ronald-Louis Ballouz, "The Brazil nut effect and its application to asteroids," Mon. Not. R. Astron. Soc., vol. 443, no. 4 (October 1, 2014), pp. 3368-3380. A PDF file is available here, and also at arXiv.