
Seeing Infrared
January 19, 2015 Infrared light has been technologically useful for many things. Short-range data communication by infrared light in the 850–900 nanometer range is used in many devices, including the ubiquitous remote control. As in many consumer technologies, advances in functionality were obtained though standardization; in this case, the Infrared Data Association (IrDA) standard. | A collection of infrared remote control devices in my house. The Panasonic remote for our legacy videocassette recorder is seldom used. (Photo by author). |
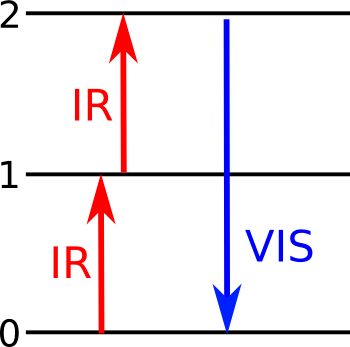 | Energy level diagram for upconversion via excited state absorption. An electron at a ground state (0) is excited to a first (1) energy level by an infrared photon, and a higher, second (2) energy level by another photon. When it reverts back to its ground state, visible light is emitted. (Illustration by the author, rendered with Inkscape.) |
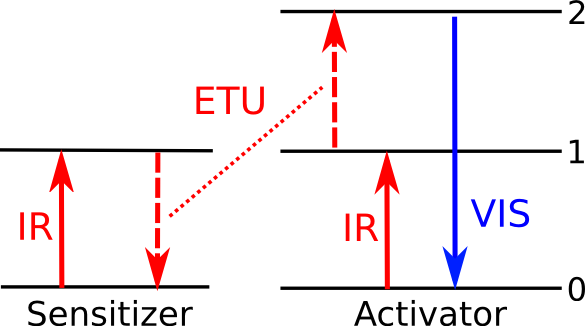 |
Energy level diagram for upconversion via energy transfer. An electron at a ground state (0) in an activator ion is excited to a first (1) energy level by infrared light, and a second (2) energy level with help from non-radiative energy transfer from a sensitizer ion. When it reverts back to its ground state, visible light is emitted. (Illustration by the author, rendered with Inkscape.) |
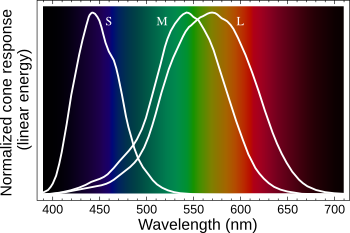 | Visual response of the retinal S, M and L cone cells that define color vision. (Modified Wikimedia Commons image.) |
"They were able to see the laser light, which was outside of the normal visible range, and we really wanted to figure out how they were able to sense light that was supposed to be invisible."[5]Vinberg and his colleagues combed the scientific literature to see what conditions resulted in human observation of infrared, and they conducted psychophysical experiments that proved the effect.[4-5] In their experiments, the research team beamed pulses of infrared light into the eyes of their subjects. By varying the pulse width, but keeping the total number of photons in each pulse constant, they found that a rapid influx of photons resulted in two infrared photons teaming to produce a green light photon.[5] The sensitivity increased at wavelengths above 900 nm. Doubled 900 nm light would be at 450 nm, which is at the lower range of optical sensitivity. The effect displayed a quadratic dependence on laser power, an indicator of a nonlinear optical process.[4] Other experiments showed that infrared light causes a photoisomerization of a model chromophore compound, which indicates that human perception of infrared light occurs by such an isomerization of visual pigments.[4] This strange visual phenomenon might have diagnostic value for diseases such as macular degeneration, since it would allow physicians to stimulate the retina in a new way.[5] This research was funded by the National Institutes of Health (NIH) and other agencies.[5]
References:
- N. Menyuk, K. Dwight and J.W. Pierce, "NaYF4 : Yb,Er—an efficient upconversion phosphor," Appl. Phys. Lett., vol. 21, no. 4 (August 15, 1972), pp. 159 ff. http://dx.doi.org/10.1063/1.1654325
- Yanmin Yang, Chao Mi, Fuyun Jiao, Xianyuan Su, Xiaodong Li, Linlin Liu, Jiao Zhang, Fang Yu, Yanzhou Liu, and Yaohua Mai, "A Novel Multifunctional Upconversion Phosphor: Yb3+/Er3+ Codoped La2S3," Journal of the American Ceramic Society, vol. 97, no. 6 (June, 2014), pp. 1769–1775.
- Ezra Lucas Hoyt Cates, Dissertation: Development of Visible-to-Ultraviolet Upconversion Phosphors for Light-Activated Antimicrobial Technology, Georgia Institute of Technology, May, 2013.
- Grazyna Palczewska, Frans Vinberg, Patrycjusz Stremplewski, Martin P. Bircher, David Salom, Katarzyna Komar, Jianye Zhang, Michele Cascella, Maciej Wojtkowski, Vladimir J. Kefalov, and Krzysztof Palczewski, "Human infrared vision is triggered by two-photon chromophore isomerization," Proc. Natl. Acad. Sci., (Online Early Edition, December 1, 2014), doi:10.1073/pnas.1410162111.
- Jim Dryden, "The human eye can see 'invisible' infrared light," Washington University School of Medicine Press Release, December 1, 2014.