
Plasma Cushion
September 14, 2015 Love may make the world go 'round, but electrons are what hold it together. Electrons bond atoms together in solids, and matter is affected by electrical forces in many ways. In my graduate research, I was introduced to two unusual ways in which electricity can modify materials. These were electrical discharge machining and electromigration. HfV2 is an intermetallic compound formed from hafnium and vanadium, and it's interesting because it's a superconductor, and it absorbs hydrogen.[1] It's also an extremely hard material that can't be cut by a hacksaw. The way that we prepared specimens of HfV2 for measurement was to slice them in an electrical discharge machine. Electric current pulses between a razor blade and our alloy in a tank of dielectric liquid made straight cuts at a rate of about a millimeter/hour. You need more than just smaller transistors to make a smaller integrated circuit. The transistors would be useless without the "wires" that link them together. The "wires" in this case are planar deposits of a conducting metal, and the metal used for these interconnects was originally pure aluminum, since it's easy to deposit. When the cross-sectional area of a current-carrying wire becomes small, the current density, which is the number of electrons moving though the area in a given time, becomes large. | There were a modest 4.3 million transistors on this chip in 1997. Advanced CPUs now have 5 billion. Photo of an AMD K5 PR150 microprocessor die by Pauli Rautakorpi, via Wikimedia Commons.) |
in which A and n are model parameters, j is the current density, Q is an activation energy, k is the Boltzmann constant, and T is the absolute temperature. This, of course, is a typical Arrhenius law model so useful in many problems, with the values of A, n, and Q determined from experiment. Ideally, n = 2. The model is important, since testing at elevated temperature will predict performance at operating temperature. Electric fields will affect solids suspended in liquids by the electrorheological effect in which small, non-conducting particles in an insulating fluid will align themselves with the electric field. The alignment arises from the polarizability of the particles, and the polarized particles stick together like magnets. I wrote about this effect in an earlier article (Electrorheology, April 13, 2012) The electrorheological effect was discovered by Willis M. Wlnslow, who patented some applications in 1947, and published a paper in the Journal of Applied Physics in 1949.[2-3] Winslow was able to attain a reversible shear resistance of several hundred grams per square centimeter. Unfortunately, electric field strengths of the order of a kilovolt per millimeter are required.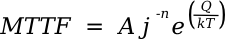
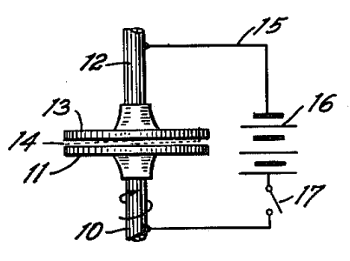 | Figure 1 of US Patent No. 2,417,850, "Method and Means for Translating Electrical Impulses into Mechanical Force," Willis M. Winslow, March 25, 1947. (Via Google Patents). [2] |
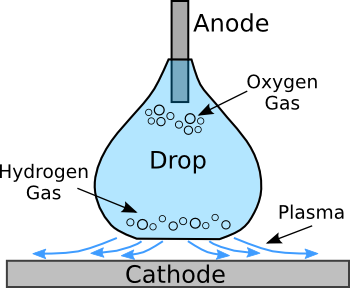 | Schematic diagram of a plasma-levitated droplet. The droplet is a dilute solution of hydrochloric acid. Electrolysis produces oxygen at the anode, and hydrogen at the cathode. (Illustration by the author using Inkscape.) |
 | Plasma-levitated droplet. The blue glow of the plasma below the dilute hydrochloric acid solution, generated at somewhat more than 50 volts, is clearly see. (Photograph: Cedric Poulain, et al / CEA.) |
References:
- P. Duffer, D.M. Gualtieri, and V.U.S. Rao, "Pronounced Isotope Effect in the Superconductivity of HfV2 Containing Hydrogen (Deuterium)," Phys. Rev. Lett., vol. 37, no. 21 (November 22, 1976), pp. 1410-1413.
- Willis M. Winslow, "Method and Means for Translating Electrical Impulses into Mechanical Force," US Patent No. 2,417,850, March 25, 1947.
- Willis M. Winslow, "Induced fibration of suspensions," Journal of Applied Physics, vol. 20, no. 12 (December 1, 1949), pp. 1137-1140.
- Carlos S. Orellana, Jinbo He and Heinrich M. Jaeger, "Electrorheological response of dense strontium titanyl oxalate suspensions," Soft Matter, 2011, vol. 7, no. 18 (2011), pp. 8023-8029; PDF file available, here.
- Hiroshi Matsukawa, Summary of "Friction Control of a Gel by Electric Field in Ionic Surfactant Solution," J. Phys. Soc. Jpn. Online News and Comments, June 10, 2010.
- Cedric Poulain, Antoine Dugue, Antoine Durieux, Nader Sadeghi, and Jerome Duplat, "The plasma levitation of droplets," Applied Physics Letters, vol. 107, Document No. 064101 (August 11, 2015), http://dx.doi.org/10.1063/1.4926964. This is an Open Access publication with a PDF file available, here.
- Droplets levitate on a cushion of blue light, American Institute of Physics Press Release, August 11, 2015.
- Willis M. Winslow, "Induced fibration of suspensions," Journal of Applied Physics, vol. 20, no. 12 (December 1, 1949), pp. 1137-1140.