
Nitrous and Nitric Oxides
August 3, 2015 Mathematics, like physics and chemistry, is subdivided into many specialties. Generally, practitioners in any one of these has only a cursory understanding of what's happening in the others. While most inorganic chemists can render the periodic table of the elements from memory, this would be a hard task for an organic chemist. Likewise, condensed matter physicists can't recite the members of the particle zoo discovered by their particle physics counterparts. Likewise, mathematics is fragmented into many fields. You can view a list of the fields of mathematics on the arXiv topic page. While most mathematicians are knowledgeable just in their specialty area, Paul Erdős was a true "poly-math," since he made contributions to so many fields of mathematics. As was his custom, Erdős mostly collaborated with others, thereby inspiring the concept of the Erdős number, which is the "collaborative distance" between Erdős and another person. A coauthor of a paper with Erdős has an Erdős number of one, while a coauthor of a coauthor with Erdős has an Erdős number of two, etc. | Paul Erdős and ten year old Terence Tao in 1985. Tao, whose work I presented in a previous article, has an Erdős number of two. (Via Wikimedia Commons.) |
 | An engraving entitled, "Chemical Recreations." This shows nitrous oxide inducing laughing and dancing. English chemist, Joseph Priestley (1733-1804), discovered nitrous oxide in 1772, and named it phlogisticated nitrous air. He prepared it by heating iron filings and nitric acid. (Illustration from Wellcome Images, a website operated by Wellcome Trust, a global charitable foundation based in the United Kingdom, via Wikimedia Commons.) |
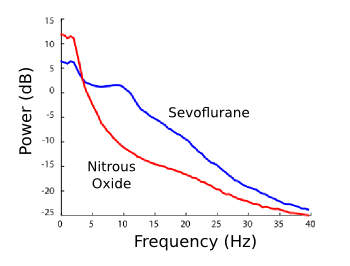 | EEG spectra under a sevoflurane ether (blue) and nitrous oxide (red) anesthetic. The excess of low frequency delta waves is clearly see. (Fig. 2c of ref. 2, modified for clarity, licensed under a Creative Commons License.) |
"We literally watched it and marveled, because it was totally unexpected... Nitrous oxide has control over the brain in ways no other drug does."[3]The reason that the delta waves are increased just at the start of dosing, and then fall-off, is unexplained. The researchers suspect a rapid habituation or desensitization process.[3] The research team is presently investigating how all principal anesthetics and anesthetic combinations affect the EEG signatures.[3] The nitric oxide cousin of nitrous oxide also has a medical application. Inhaled nitric oxide relaxes pulmonary vessels without causing a systemic drop in blood pressure. The effect, discovered in 1999, is now used to treat about 35,000 U.S. patients in hospitals each year, some of whom are newborns with a condition called persistent pulmonary hypertension of the newborn (PPHN).[5] Nitric oxide therapy for PPHN, which takes about five days, costs about $14,000. Much of the cost arises from distribution and handling of gas cylinders, and the associated delivery and monitoring devices.[4-5] Another team of researchers at the Massachusetts General Hospital of Harvard Medical School (Boston, Massachusetts) has developed a simple device that uses a pulsed electrical discharges to produce nitric oxide in the therapeutic range of 5-80 parts per million at carrier gas flow rates of 0.5-5 liters/minute. The nitric oxide is produced from the air, or from gas mixtures of 90% O2 and 10% N2 (see photo).
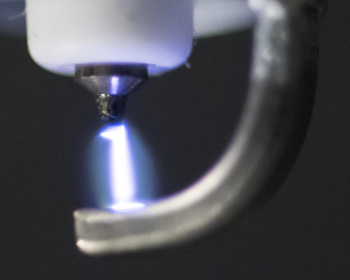 | An electrical spark generating therapeutic nitric oxide from the air Iridium was found to be the best electrode material for this application. (Photo by Brian Wilson, Massachusetts General Hospital Photography Department.)[5] |
References:
- Laughing Gas (1914, Charles Chaplin, Director) on the Internet Movie Database.
- Kara J. Pavone, Oluwaseun Akeju, Aaron L. Sampson, Kelly Ling, Patrick L. Purdon, and Emery N. Brown, "Nitrous oxide-induced slow and delta oscillations," Clinical Neurophysiology, Article in Press, DOI: http://dx.doi.org/10.1016/j.clinph.2015.06.001. This is an open access publication with a PDF file available here.
- Uncovering the mechanism of our oldest anesthetic, MIT Press Release, July 6, 2015.
- Binglan Yu, Stefan Muenster, Aron H. Blaesi, Donald B. Bloch, and Warren M. Zapol, "Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy," Science Translational Medicine, vol. 7, no. 294 (July 1, 2015), pp. 294ff., DOI: 10.1126/scitranslmed.aaa3097.
- Mass. General team generates therapeutic nitric oxide from air with an electric spark, Massachusetts General Hospital Press Release, July 6, 2015.