Materials of the Deep
April 13, 2015
The
open sea has always had an air of mystery. Just as our
ancestors imagined strange underworlds beneath the
surface of Earth, they imagined strange things existing in the great
ocean depths where they couldn't venture. That's why older
maps had images of
strange sea creatures and captions such as, "Here, there be monsters."
Despite the perceived
dangers, men were still willing to set out to sea to harvest its wealth of
food stocks, and also its
natural material resources. One of the
chemicals harvested from the sea in
antiquity was
Tyrian purple, also known as royal purple. This extract from the
sea snail,
Bolinus brandaris, is a
natural dye. It was expensive to produce; thus, the "royal" appellation.
Some marine materials are especially easy to harvest since you can find them on land.
Limestone, a very useful building material, is formed from the the
skeletal remains of
marine organisms, such as
Foraminifera. The
metamorphic form of limestone,
marble, was an important
structural material in antiquity. The skeletal remains are chemically
calcium carbonate (CaCO
3), but in two
different crystalline forms,
calcite and
aragonite.
The question naturally arises as to why
nature would have its marine creatures produce two different forms of calcite as a skeletal material, especially since the aragonite form is a
metastable form that's more
soluble, especially in
acidic waters. As a team of
scientists from the
Massachusetts Institute of Technology (Cambridge, Massachusetts) and
Lawrence Berkeley National Laboratory (Berkeley, California) write in a recent issue of the
Proceedings of the National Academy of Science, the choice of
crystal habit is actually an "accident of birth."[1-2]
"Birth" in the case of
crystal growth is the
nucleation phase when the first few
atoms of material arrange themselves into the template on which subsequent crystal will grow. As the scientists discovered, the
magnesium concentration in the water is the determining factor. The
calcium-to-magnesium
ratio affects the
surface energy of the nucleating crystals, and beyond a certain ratio, aragonite is favored over calcite.[1-2]
Calcite is a
trigonal crystal with
unit cell dimensions, a = 4.99
pm, and c = 1706 pm, while aragonite is an
orthorhombic crystal with unit cell dimensions, a = 495 pm, b = 796 pm, and c = 574 pm. It's easy to see why magnesium substituting for calcium would have a large affect on crystal energy. The
ionic radius of Ca
2+ is 114 pm, while the ionic radius of Mg
2+ is 86 pm, so substitution of magnesium for calcium would lead to considerable lattice
strain.
The metastable forms of
elements and
compounds generally have different
properties than the stable form. One example is
diamond, a metastable form of
carbon with extreme
hardness. In the case of calcium carbonate, the aragonite phase is more soluble, which has implications for the
natural sequestration of
carbon dioxide in seawater.[1-2] The aragonite shells of marine life are more vulnerable to ocean acidification, which is an effect of
climate change.[1]
The
experimental measurement of surface energy is difficult, so the
research team turned to atomic-level
calculations. The calculations revealed that magnesium concentration causes the surface energy of calcite to increase to a point at which nucleation is diminished by
orders of magnitude for that crystal phase. While calcite growth is impeded, the metastable aragonite phase is favored.[2] This calculation is general enough that it could be applied to the crystallization of other compounds from
solution.[2]
A scientific understanding of how metastable phases are created from solutions would be beneficial. Faster dissolving
pharmaceuticals would be useful, as would more stable
photocatalysts. The research team is developing their atomic model into a method to predict other material properties, such as chemical
reactivity,
electrical conductivity, and hardness. This research was funded by the
U.S. Department of Energy and the
National Science Foundation.[2]
Another research study on a material from the sea,
limpet teeth, has just been published as an
open access paper in the
Journal of the Royal Society Interface.[3] In this study, it was found that limpet teeth are formed from the strongest natural material found to date, stronger than
spider silk and stronger than many man-made materials.[3-7] I wrote about spider silk in two previous articles,
Spider Silk, March 12, 2012 and
Spider Silk Mechanics, February 15, 2013.
Limpet teeth are actually part of the limpet
tongue, called a
radula, and they are used to scrape food from
rock surface. For such a purpose, these teeth need to be strong, so strong that they actually scrape away some rock, which is swallowed and is excreted as hardened blocks in their
fecal pellets.[4] Limpet teeth are made from a
composite of
protein material and the
mineral,
goethite (α-FeO(OH)) that exists as
needle-like crystals. The composite is structured much like
carbon fiber reinforced plastic.[4]
Since limpet teeth are very small, of the order of 100
μm,
mechanical measurement of their strength is difficult. The research team used
in situ atomic force microscopy for
tensile strength measurements, after first
milling teeth to the typical "dog bone" specimen shape in which the central area was a hundred times thinner than a
human hair.[3-4] The specimens were pulled inside the atomic force microscope until they broke.[4]
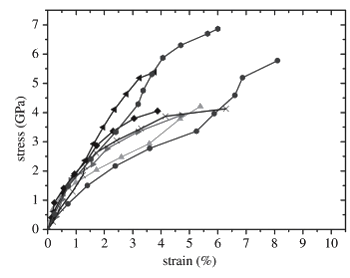
Although the measurement is difficult, the interpretation of the
data is straightforward. The tensile strength that they found was in the range 3.0 to 6.5
GPa, it was independent of sample size, and about five time greater than the tensile strength of spider silk.[3-5] The high strength is attributed to the
nanoscale dimension of the goethite reinforcing fibers. These fibers have a diameter less than the
critical flaw size as developed in
Griffith's fracture theory of
glass, and this suggests that the material was optimized by
natural selection for high strength.[3]
Says the lead
author of the limpet study,
Asa Barber, a
professor at the
University of Portsmouth (Portsmouth, UK),
“Biology is a great source of inspiration when designing new structures but with so many biological structures to consider, it can take time to discover which may be useful.”[6]
References:
- Wenhao Sun, Saivenkataraman Jayaraman, Wei Chen, Kristin A. Persson, and Gerbrand Ceder, "Nucleation of metastable aragonite CaCO3 in seawater," Proc. Natl. Acad. Sci. (published ahead of print, March 4, 2015), doi:10.1073/pnas.1423898112.
- David L. Chandler, "Mystery solved: Why seashells’ mineral forms differently in seawater," MIT Press Release, March 2, 2015.
- Asa H. Barber , Dun Lu , and Nicola M. Pugno, "Extreme strength observed in limpet teeth," J. R. Soc. Interface, vol. 12 (February 15, 2015), article no. 20141326, DOI: 10.1098/rsif.2014.1326. This is an open access publication with a PDF file available here.
- Jonathan Webb, "Limpet teeth set new strength record," BBC News, February 18, 2015.
- Strongest material known to man? A limpet's tooth, Telegraph (UK), February 18, 2015.
- Scientists find strongest natural material, University of Portsmouth Press Release, February 18, 2015.
- Limpets' teeth consist of the strongest biological material, scientists say, BBC, February 18, 2015.